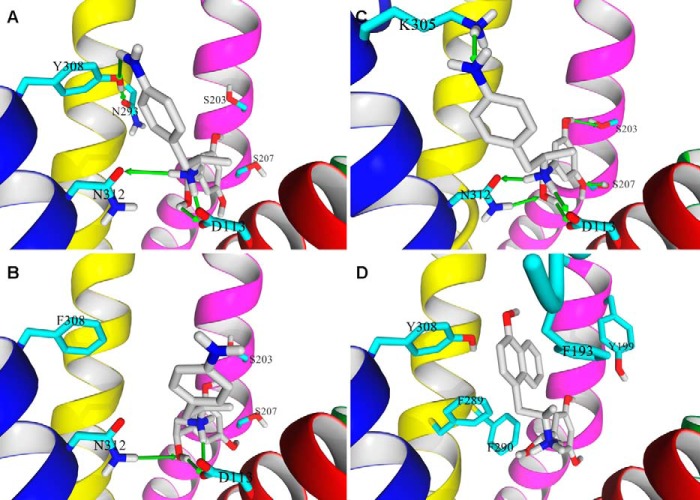
FIGURE 9.
Binding poses of (R,R′)-aminoFen and (R,R′)-MNFen docked to the β2-AR models. A, docking of (R,R′)-aminoFen to the carazolol-bound β2-AR model (PDB entry 2RH1), and B, to the β2-AR Y308F mutant. The 4′-amino group of (R,R′)-aminoFen forms a HB with the Tyr-308 residue of β2-AR (green arrow), and the interaction is lost in the β2-AR Y308F mutant. C, docking of (R,R′)-aminoFen to a BI-167107 and Gs protein-bound conformation of β2-AR (PDB entry 3SN6). The location of the docked molecule highly resembles the orientation of co-crystallized agonist, BI-167107, as both molecules share significant structural similarities. The agonists form a network of analogous HB interactions with the receptor residues Ser-203, Ser-207, Asn-312, and Asp-113. An additional interaction can be observed between the 4′-amino moiety of the ligand and Lys-305 residue. D, docking of (R,R′)-MNFen to a carazolol-bound conformation of β2-AR (PDB entry 2RH1). Aromatic residues in the ligand binding pocket able to form π-π interactions with the naphthyl moiety of the ligand are shown. Ligand molecule is rendered in atom-type color-coded stick mode, and five essential TM helices of target β2-AR model are shown and colored as follows: TM3, magenta; TM4, green; TM5, red; TM6, yellow; and TM7, blue.