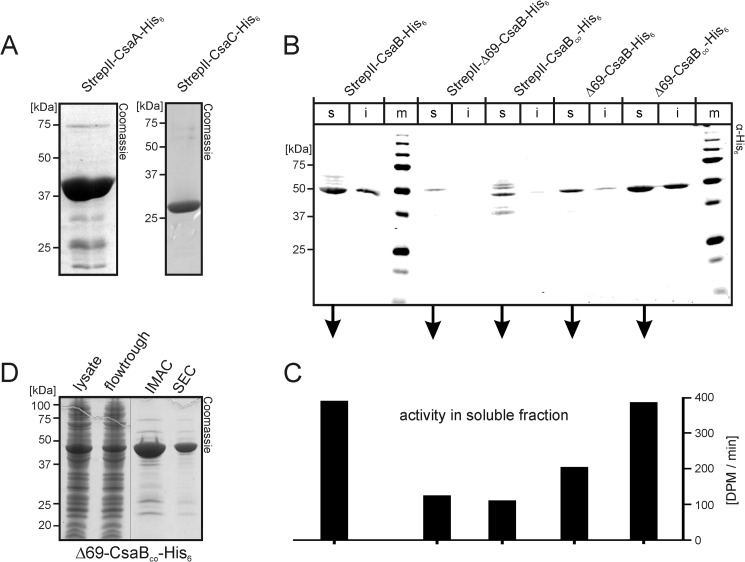
FIGURE 2.
Production of recombinant enzymes. A, Coomassie-stained SDS-PAGE of purified StrepII-CsaA-His6 (left panel) and StrepII-CsaC-His6 (right panel). B, to select the construct most suited for the production of active recombinant CsaB, the wild type and a codon-optimized (CsaBco) version of the CsaB sequence were cloned (full-length or after N-terminal truncation, Δ69) to produce proteins with tags on both (StrepII and His6) or only one end (His6), as indicated. Transformed bacteria were lysed, separated into soluble (s) and insoluble (i) fractions, and fractions were separately run on PAGE. After transfer onto nitrocellulose, the blot was developed with an anti-penta-His antibody. m, marker. C, soluble fractions were used to measure CsaB activity in a radioactive incorporation assay. D, Coomassie-stained gel demonstrating the purification result for Δ69-CsaBco-His6 (IMAC, immobilized metal ion affinity chromatography; SEC, size exclusion chromatography).