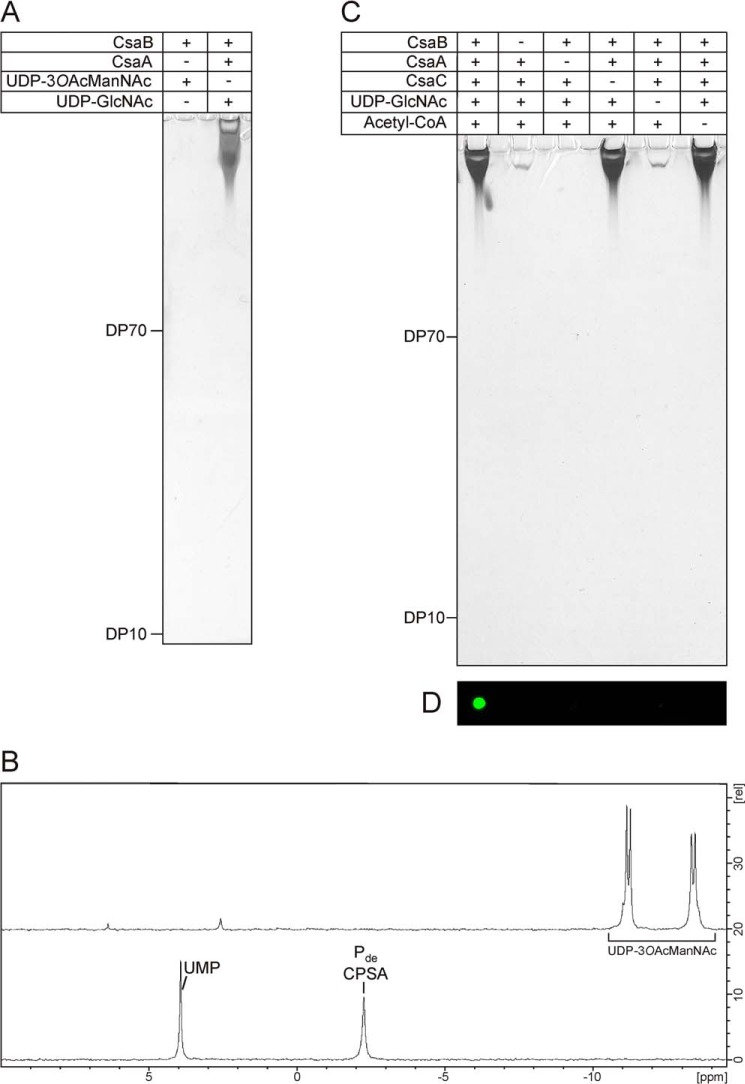
FIGURE 8.
A one-pot reaction for the synthesis of immunologically active O-acetylated CPSA. A, UDP-3OAcManNAc was chemically synthesized to test if O-acetylated CPSA could be obtained in a one-step reaction. Analysis of the reaction by high percentage PAGE followed by combined Alcian blue/silver staining did not reveal any product signals (left lane), whereas long CPSA was obtained in the control reaction (right lane). B, 31P NMR analysis of the samples revealed that UDP-3OAcManNAc (top spectrum) was not consumed by CsaB, whereas product signals were detected in the control (bottom spectrum). C, in vitro synthesis of O-acetylated and non-O-acetylated CPSA using all enzymes CsaA-C in a one-pot reaction. To control product formation, substrates and enzymes were added as indicated. After a 2-h incubation, products were displayed on high percentage PAGE by a combined Alcian blue/silver staining. Long chains were synthesized in all reactions containing CsaA, CsaB, and UDP-GlcNAc. D, only products obtained in the reaction where CsaC and acetyl-CoA were present could be detected with mAb 932 specifically directed against the CPSAOAc.