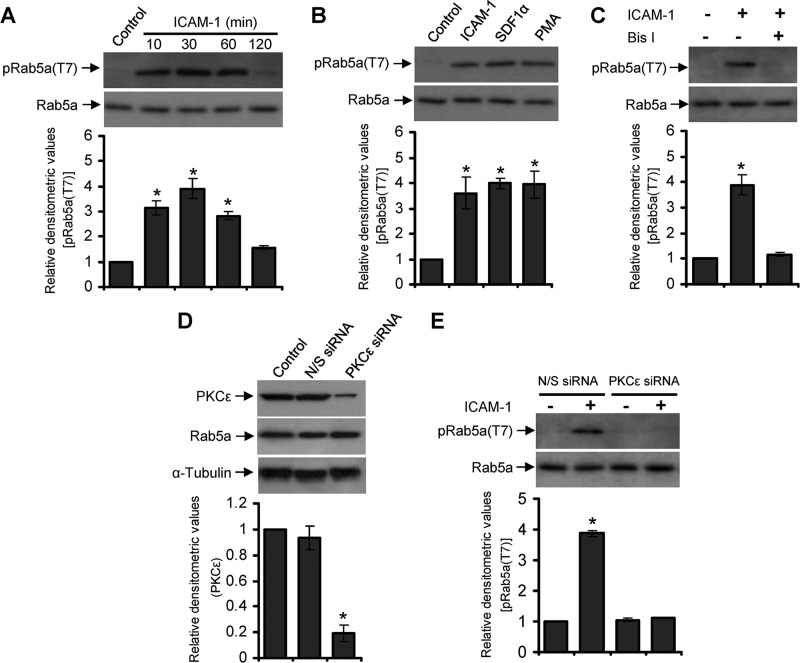
FIGURE 4.
Rab5a undergoes PKCϵ-dependent phosphorylation on Thr-7 in migrating T-cells. A, human primary PBL T-cells were allowed to migrate via LFA-1 stimulation by incubating on ICAM-1-coated plates for 10, 30, 60, or 120 min and lysed. Cell lysates were analyzed by Western immunoblotting using the crude anti-phospho Rab5a(Thr-7) antiserum (pRab5a(T7)). B, PBL T-cells were left unstimulated or stimulated with ICAM-1 for 30 min, SDF-1α for 2 min, or phorbol 12-myristate 13-acetate (PMA) for 30 min and lysed. Cell lysates were analyzed by Western immunoblotting using the crude pRab5a(Thr-7) antiserum. C, PBL T-cells were untreated or pretreated with PKC inhibitor bisindolylmaleimide I for 30 min, stimulated with ICAM-1 for additional 30 min, and lysed. Cell lysates were analyzed by Western immunoblotting using the crude anti-pRab5a(Thr-7) antiserum. D, PBL T-cells were nucleofected with nonspecific siRNA (N/S siRNA) or siRNA against PKCϵ (PKCϵ siRNA) and then lysed after 72 h. Cell lysates were Western-immunoblotted with anti-PKCϵ antibody. E, PBL T-cells nucleofected with nonspecific siRNA or PKCϵ siRNA were unstimulated or stimulated with ICAM-1 for 30 min and Western-immunoblotted using the crude anti-pRab5a(Thr-7) antiserum. All blots were stripped and re-probed with anti-Rab5a or α-tubulin as indicated. Densitometry analysis of the blots were performed and presented. Results are representative of at least three independent experiments. *, p < 0.01.