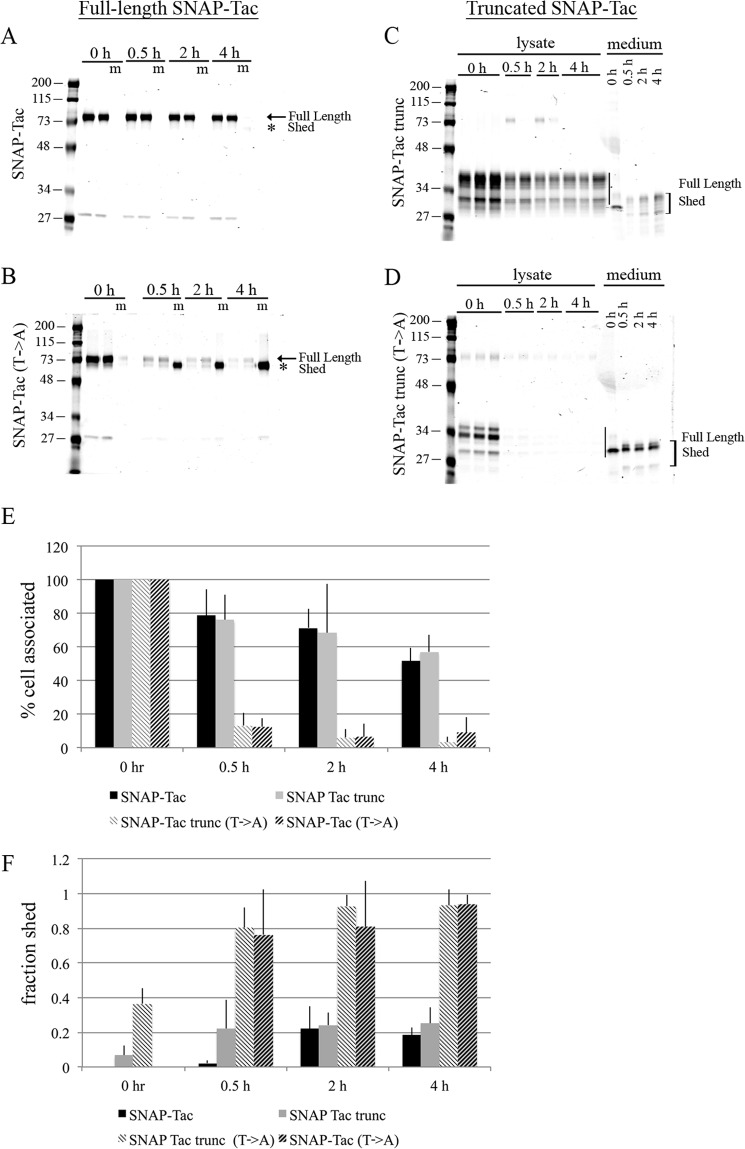
FIGURE 5.
Mutation of O-linked glycosylation results in shedding of SNAP-Tac into the medium. HeLa cells were transfected with SNAP-Tac, SNAP-Tac(T→A), SNAP-Tac trunc, or SNAP-Tac trunc(T→A) and labeled with BG-800 for 30 min on ice in serum-free DMEM. Cells were chased for 30 min, 2 h, and 4 h in serum-free DMEM containing excess BG-NH2. Media and lysates were collected and run on SDS-polyacrylamide gels. Duplicate or triplicate independent lysate samples were loaded in individual lanes. For the media samples only, one sample is loaded per time point. Coomassie staining was used to determine total protein loaded. A–D, representative gels from the indicated full-length or truncated constructs. The full-length proteins and shed products are indicated (in A and B, m represents medium). In B, the fourth lane is empty. Quantification of cell-associated (normalized to time 0) (E) and shed (the fraction of the total signal shed into the medium; see “Experimental Procedures”) (F) SNAP-Tac proteins is shown. Bar graphs represent data averaged from three independent experiments ±S.D. (error bars). The migration of prestained molecular mass markers (in kDa) is shown to the left of each panel.