FIGURE 3.
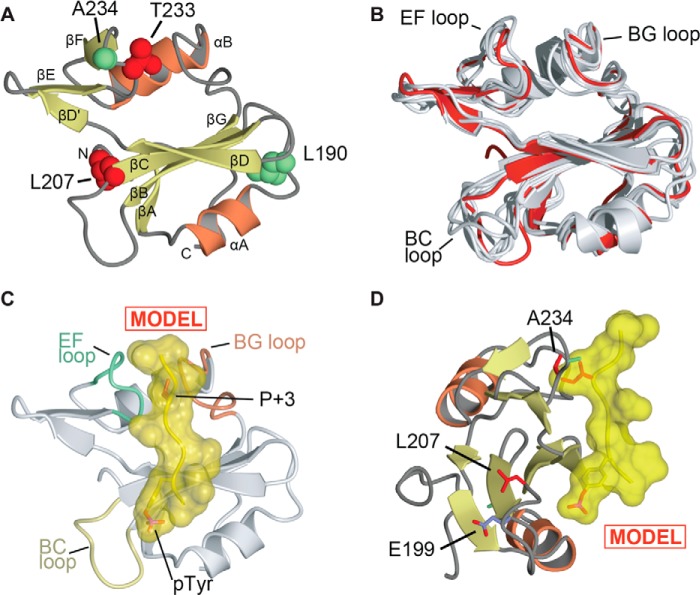
Structural analysis of Arg SH2 domain. A, overall view of the crystal structure of the Arg SH2 domain. α-Helices and β-strands are labeled. Arg residues Leu190 and Ala234 are shown as spheres and colored green. Arg residues Leu207 and Thr233 are shown as spheres and colored red. The structure is deposited in the Protein Data Bank under accession code 4EIH. B, superposition of determined structures of Arg and Abl SH2 domains. Arg SH2 domain (this study) is colored red, previously determined structures are shown in gray (PDB codes 1OPK, 1OPL, 1AB2, 2ABL, 2ECD, 2FO0, and 3K2M) (22, 31–34). The superposition was conducted using Topp (25). C and D, model of phosphotyrosine peptide binding to Arg SH2 domain. The model was generated by superposition of Arg SH2 with the Lck SH2-peptide (EPQpYEEIPIYL) complex (35). C, shows only the Arg SH2 domain and the location of the superposed peptide (in yellow). Structural diagrams generated using CCP4MG (48). D, shows an orientation rotated 90° with Arg residues Leu207, Thr233, and Glu199 shown in stick format.