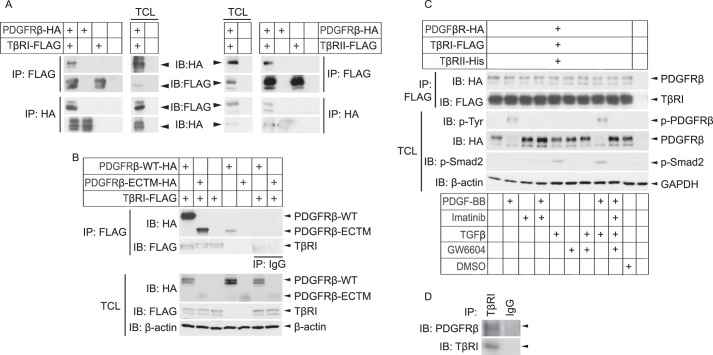
FIGURE 1.
PDGFRβ forms a physical complex with TβRI and -II. A and B, Cos1 cells were transiently transfected with PDGFRβ-HA (A and B), TβRI-FLAG (A, left panel), TβRII-FLAG (A, right panel), a truncated PDGFRβ (PDGFRβ-ECTM-HA) expressing only the extracellular and transmembrane parts of the receptor (B), and/or empty vectors. After 48 h, lysates were prepared and subjected to immunoprecipitation (IP) using FLAG or HA antibodies, and proteins were separated by SDS-PAGE. Total cell lysates were run in parallel. Immunoblotting (IB) was performed with FLAG and HA antibodies; β-actin was used as a loading control. C, Cos-1 cells were transiently transfected with PDGFRβ-HA, TβRI-FLAG, TβRII-His, and/or empty vectors. After 24 h, cells were starved for another 24 h and then stimulated with combinations of PDGF-BB (7 min, 10 ng/ml), PDGFRβ inhibitor imatinib (1 h, 5 μm), TGFβ (1 h, 1 ng/ml), TβRI inhibitor GW6604 (2 h, 6 μm), DMSO control (2 h), or starvation medium alone. Cells were lysed, and the lysate was subjected to immunoprecipitation with FLAG antibodies, and proteins were separated by SDS-PAGE. Total cell lysates (TCL) were run in parallel. Proteins were detected by immunoblotting with specific antibodies against the HA and FLAG tags, phosphotyrosine, phospho-Smad2, and β-actin. D, human dermal primary fibroblasts were immunoprecipitated using a TβRI antibody or IgG isotype control, followed by immunoblotting with specific PDGFRβ and TβRI antibodies. Representative data from at least three independent experiments are shown.