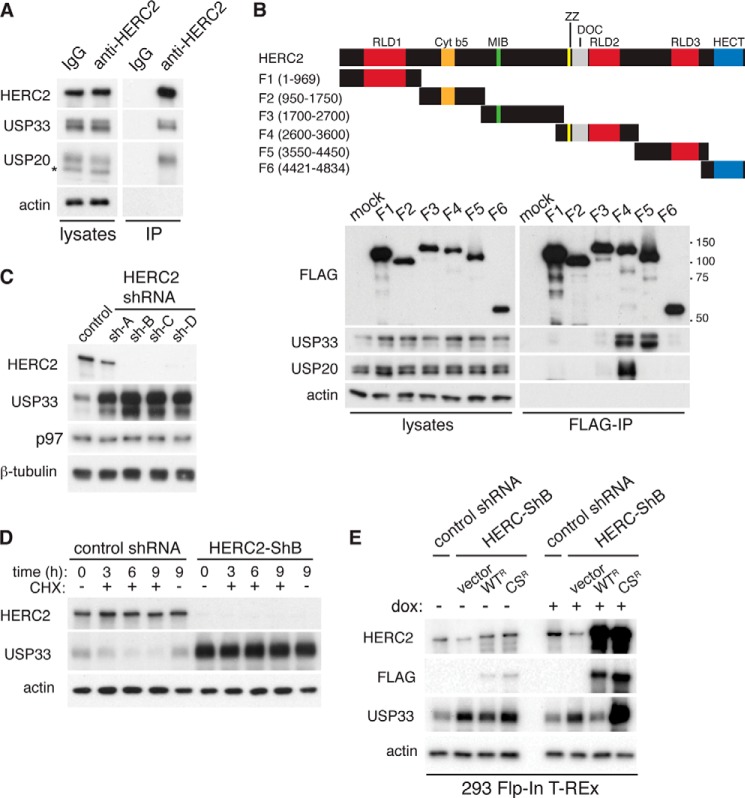
FIGURE 4.
HERC2 is an E3 ligase important for the degradation of USP33. A, co-immunoprecipitation (IP) of endogenous USP33 and USP20 with endogenous HERC2. Cell lysates were prepared from HeLa cells and immunoprecipitation experiments were performed using control or anti-HERC2 antibody. Co-immunoprecipitated proteins were detected by immunoblotting. Asterisk, nonspecific band detected by the anti-USP20 antibody. B, characterization of HERC2-USP33 interaction. FLAG-tagged HERC2 fragments were transiently transfected into HeLa cells, and anti-FLAG antibody was used to immunoprecipitate the HERC2 fragments. Co-immunoprecipitated endogenous proteins were detected by immunoblotting. DOC, doxycyline. C, accumulation of USP33 upon knockdown of HERC2. Control (nontargeting) shRNA or four independent shRNAs targeting different regions of the HERC2 transcript were expressed by retroviral transduction in HeLa cells. Cell lysates were isolated and analyzed by immunoblotting. D, analysis of USP33 degradation upon HERC2 knockdown. HeLa cells transduced with retrovirus encoding a control (nontargeting) shRNA or an shRNA against HERC2 (HERC2-ShB) were treated with 100 μg/ml cycloheximide (CHX) for the indicated time. Total cell lysates were isolated and analyzed by immunoblotting. E, rescue of HERC2 knockdown cells. 293 Flp-In T-Rex HERC2 knockdown cells (HERC2-ShB) expressed control vector, FLAG-tagged (RNAi-resistant) wild-type (WTR), or catalytically inactive C4762S (CSR) HERC2. To induce expression, cells were treated with 0.1 μg/ml doxycycline (dox) for 40 h, and total cell lysates were analyzed by immunoblotting.