Table 1.
Examination of chiral imidazolinium salts as catalyst precursors.[a]
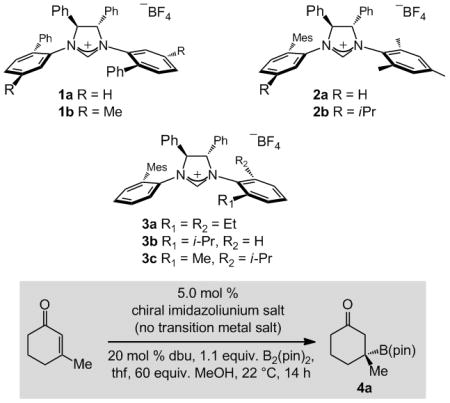
| ||||
|---|---|---|---|---|
| Entry | Imidazolinium Salt | Conv. [%][b] | Yield [%][c] | e.r.[d] |
| 1 | 1a | 53 | 46 | 84:16 |
| 2 | 1b | 96 | 85 | 84:16 |
| 3 | 2a | >98 | 79 | 88:12 |
| 4 | 2b | 68 | 54 | 67:33 |
| 5 | 3a | >98 | 74 | 91.5:8.5 |
| 6 | 3b | 91 | 82 | 90:10 |
| 7 | 3c | >98 | 90 | 96:4 |
Reactions were performed under N2 atmosphere.
Determined by analysis of 400 MHz 1H NMR spectra of unpurified mixtures (±2%).
Yields of isolated and purified products (±5%).
Determined by GC analysis (±2%); see the Supporting Information for details. dbu = 1,8-diazabicyclo[5.4.0]undec-7-ene; Mes = 2,4,6-Me3C6H2.
