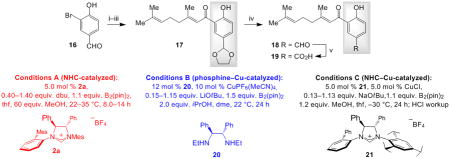
Table 2.
Comparison of different Approaches en Route to (−)-Crassinervicn Acid.[a]
| ||||
|---|---|---|---|---|
| Product | Conditions | Conv. [%][b] | Yield [%][c] | e.r.[d] |
|
| ||||
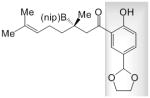 22 |
A; 0.4 equiv. dbu, 22 °C, 14 h | >98 | 63 | 84:16 |
| B; 0.15 equiv. LiOtBu, 22 °C, 24 h | 87 | 78 | 60:40 | |
| C; 0.13 equiv. NaOtBu, −30 °C, 24 h | >98 (to 23) | 82 (of 23) | 69:31 | |
|
| ||||
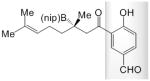 23 |
A; 0.4 equiv. dbu, 35 °C, 8.0 h | >98 | 72 | 95:5 |
| B; 0.15 equiv. LiOtBu, 22 °C, 24 h | >98 | 19 | nd | |
| C; 0.13 equiv. NaOtBu, −30 °C, 24 h | >98 | <2 | na | |
|
| ||||
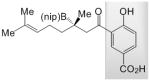 24 |
A; 1.4 equiv. dbu, 22 °C, 14 h | >98 | 70 | 88.5:11.5 |
| B; 1.15 equiv. LiOtBu, 22 °C, 24 h | >98 | <10 | nd | |
| C; 1.13 equiv. NaOtBu, −30 °C, 24 h | >98 | 22 | nd | |
Conditions: i) HO(CH2)2OH, 10 mol % pTsOH•H2O, tol., reflux, 12 h; 90% yield. ii) 3.0 equiv. tBuLi, thf, −78 °C; geranial, −78 °C, 2.0 h. iii) 1.0 mol % (n-Pr)4NRuO4, N-methylmorpholine N-oxide, CH2Cl2, 22 °C, 2.0 h. iv) 10 mol % pTsOH, acetone, 22 °C, 10 min.; 63% overall yield for three steps. v) NaClO2, NaH2PO4•H2O, tBuOH, H2O, 2-methyl-2-butene, 22 °C, 3.0 h; 82% yield. Reactions were performed under N2 atmosphere.
Determined by analysis of 400 MHz 1H NMR spectra of unpurified mixtures (±2%).
Yields of isolated and purified products (±5%).
Determined by GC analysis (±2%). See the Supporting Information for details.
