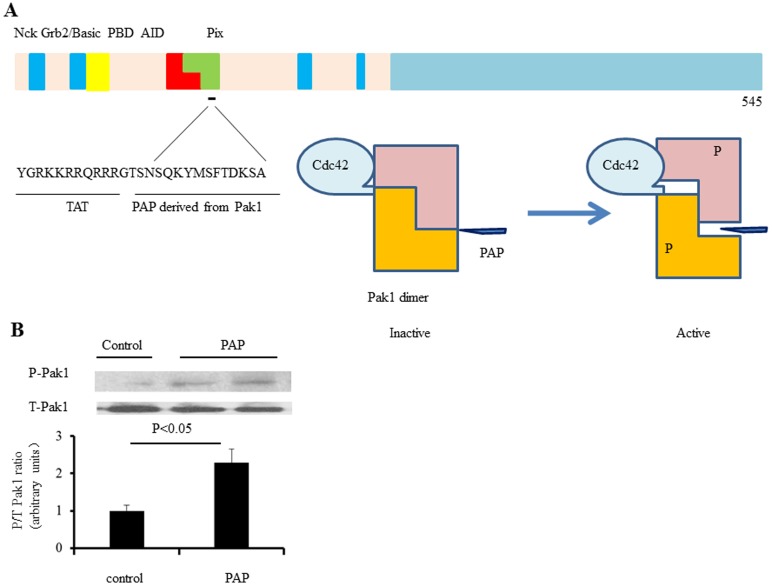
Figure 1. PAP and its interaction with Pak1.
A: Pak1 is divided into N-terminal and C-terminal halves. The N-terminal half contains p21 binding domain (PBD) followed by a kinase inhibitory domain, which overlap with each other. The proline rich motifs interact with different cellular proteins including Nck, Grb2 and Pix, etc. PAP is derived from the autoinhibitory domain linked to a TAT sequence. PAP binds to Pak1 and may activate Pak1 through attenuation of Pak1 autoinhibition in a similar way as Cdc42 and Rac1 do. B: We examined the effects of the PAP on Pak1 phosphorylation in cultured neonatal rat ventricular myocytes (NRVMs) that were treated with PAP (20 µg/ml) for 2 hours. Immunoblotting analyses of Pak1 phosphorylation indicate that Pak1 activation was induced by angiotensin II (Ang II), in NRVMs (n = 3 independent experiments).