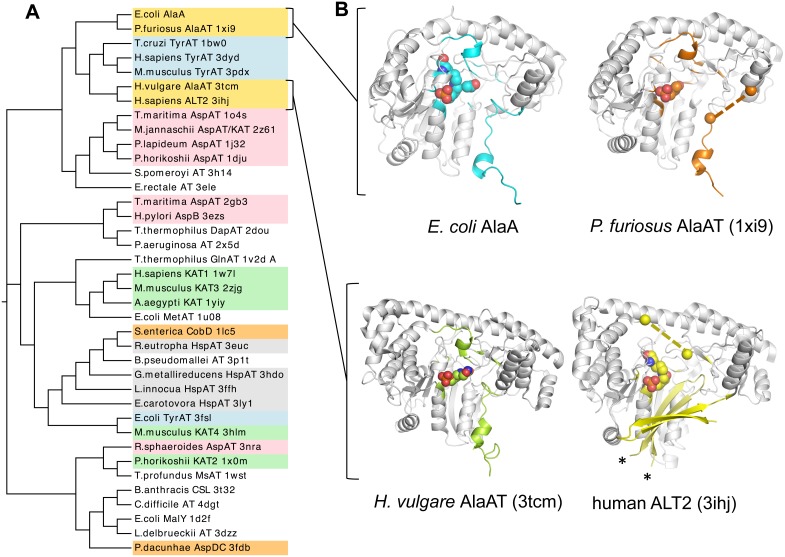
Figure 3. Comparison of AlaA with structurally homologous enzymes.
(A) Phylogenetic tree based on structure-based multiple sequence alignments of AlaA obtained from PDBeFold [58]. Functionally related enzymes are shaded in like colors; alanine transaminases in gold, tyrosine aminotransferases (TyrAT) in cyan, aspartate aminotransferases (AspAT) in pink, kynurenine aminotransferases (KAT) in green, aspartate decarboxylases (CobD, AspDC, in orange), histidinol phosphate aminotransferases (HspAT) in grey; other transaminases of unknown function or with unique substrate preferences are not shaded. (B) Cartoon representation of alanine transaminases of known structure, highlighting the overall fold structure, catalytic residues, cofactor status and N-terminal motifs of AlaA (PLP, acetate), PfAlaAT (PMP, PDB 1xi9), HvAlaAT (DCS, PDB 3tcm) and human ALT2 (PLP, PDB 3ihj). In AlaA and HvAlaAT the N-terminal H1-plug-H2 motifs are fully structured, whereas in PfAlaAT and ALT2 different segments of the N-terminal arm are disordered. The most representative PfAlaAT monomeric structure (present in three out of four copies in the crystal asymmetric unit) lacks interpretable electron density for the eight-residue segment (from Ala14 to Leu20, delimited by orange spheres) spanning the plug. In ALT2, the N-terminal 65-amino-acid residues fold into a long β-hairpin structure that swaps domain and extends toward the opposite subunit (the start and end of the swapped β-hairpin are marked with asterisks), partially covering the active site cavity and ending in a ten-residue unstructured segment (spanning Ile95 to Gln104 until the anchor Pro105 residue). This disordered region (delimited by yellow spheres) is also located over the substrate-binding pocket and therefore may have functional and structural roles akin to those of the plug motif described in AlaA and PfAlaAT.