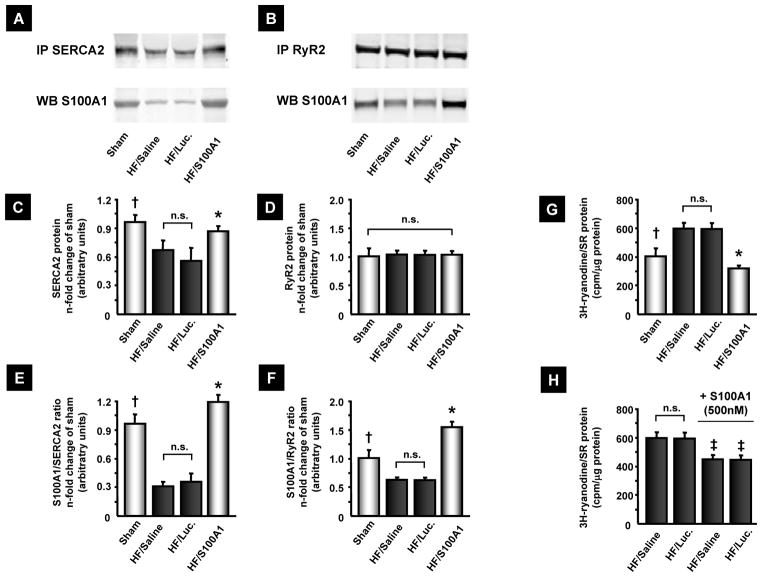
Figure 5. AAV9-S100A1 gene therapy reverses abnormal S100A1/SR target protein ratios and improves SR Ca2+ handling in failing myocardium.
(A and B) Representative immunoprecipitation (IP) for SERCA2 and RyR2 from sham, saline, AAV9-luc and AAV9-S100A1 treated myocardium and corresponding western blots for co-precipitation of S100A1 protein. (C and D) HF control groups contain significantly lower SERCA2 but equal RyR2 protein levels than sham and S100A1-treated myocardium. SR from 5 different hearts (LV anterior wall) was prepared for each group and IP’s were conducted in duplicate from each SR preparation with equal amounts of protein (300 μg). (E and F) Abnormally low S100A1/SERCA2 and S100A1/RyR2 binding ratios in HF control groups are restored after AAV9-S100A1 treatment. (G) Significantly increased 3[H]-ryanodine binding at 150 nm free Ca2+ in HF control groups indicates greater SR Ca2+ leak compared to sham and HF/S100A1. (H) Addition of human recombinant S100A1 protein to SR vesicles from HF control groups significantly reverses abnormally high 3[H]-ryanodine binding. †P<0.05 sham vs. HF/saline and HF/Luc, *P<0.05 HF/S100A1 vs. HF/saline and HF/Luc. ‡P<0.05 HF/saline and HF/luc + S100A1 protein vs. corresponding HF/saline or HF/luc. n=5 in each group. Internal control of immunoprecipitation is shown in Fig. S3.