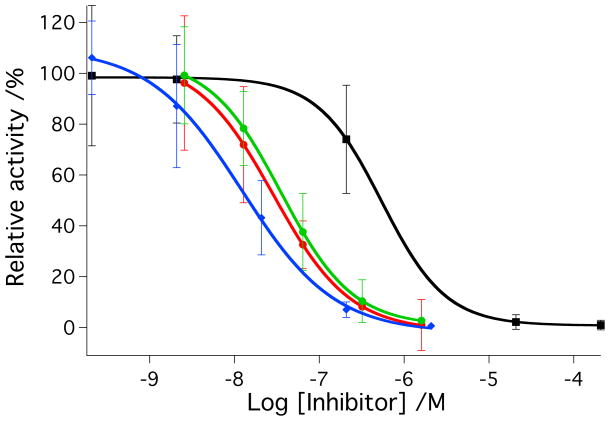
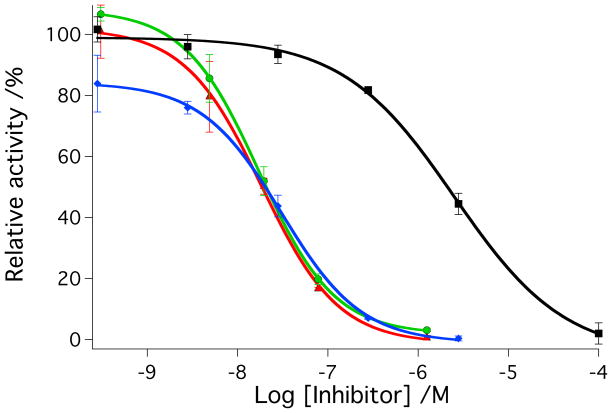
Figure 3.
A) IC50 curves for inhibitor 2 (red, dark; green, light) and cis-[Ru(bpy)2(2)2]Cl2 (4) (black, dark; blue, light) with cathepsin K. B) IC50 curves for inhibitor 3 (red, dark; green, light) and cis-[Ru(bpy)2(3)2](BF4)2 (5) (black, dark; blue, light) with cathepsin K. Enzyme activity was determined with the fluorogenic substrate Z-Gly-Pro-Arg-AMC and is expressed as a percentage, with 100% equal to the cathepsin K activity in the absence of inhibitor. Data points represent the average of triplicate wells, and error bars are standard deviations of the mean. Data are representative of three independent experiments. Conditions: 400 mM sodium acetate, pH 6.0, 4 mM EDTA, 8 mM DTT, 1% DMSO, [cathepsin K] = 2 nM, [Z-Gly-Pro-Arg-AMC] = 100 μM, 0.01% Triton X-100, 15 min irradiation for 2 and 4, 45 min irradiation for 3 and 5 with a tungsten halogen lamp (>395 nm and H2O filter, 250 W). See Experimental Section for more details.