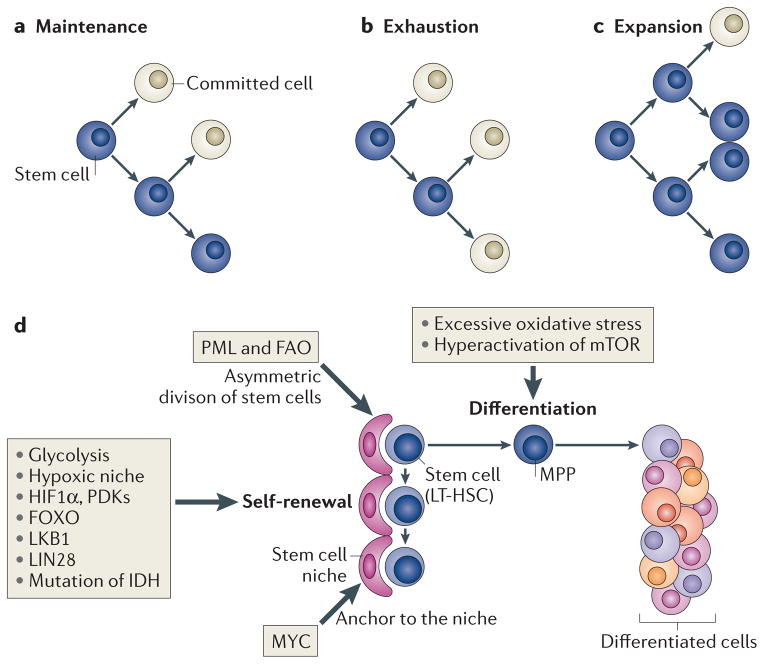
Figure 1. Two specific potentials and cell fates of stem cells.
Stem cells exhibit both self-renewal capacity and pluripotency (parts a,b,c). Asymmetric cell division has been suggested as a regulator of stem cell-fate decisions and is essential for the maintenance of the stem cell compartment (part a). Alterations in the equilibrium of self-renewal and commitment of adult stem cells can affect tissue homeostasis and can lead to stem cell exhaustion (part b) or expansion (part c). Several tissue stem cells (part d) (for example, long-term haematopoietic stem cells (LT-HSCs) in the bone marrow niche) maintain a quiescent state, as this is essential for preserving their self-renewal capacity. Many types of stem cells heavily rely on anaerobic glycolysis to maintain such a quiescent state and are more sensitive to oxidative stress. In hypoxic conditions (such as those found in the stem cell niche), the transcription factor hypoxia-inducible factor 1α (HIF 1α) promotes glycolysis as it induces the expression of pyruvate dehydrogenase kinases (PDKs), which prevent pyruvate from entering the tricarboxylic acid cycle, thus blocking mitochondrial respiration. Forkhead box O (FOXO), liver kinase B1 (LKB1) and LIN28 are crucial to maintain stem cells, and mutation of the gene encoding isocitrate dehydrogenase (IDH) leads to enhanced self-renewal capacity of HSCs. Nutrient-sensitive PI3K–AKT–mTOR pathways, Gln metabolism and fatty acid metabolism also have a crucial role in regulating the balance between quiescence and proliferation of stem cells. The boxes indicate how or which potentials of stem cells are regulated by these factors. FAO, fatty acid oxidation; MPP, multipotent progenitor cell; PML, promyelocytic leukaemia.