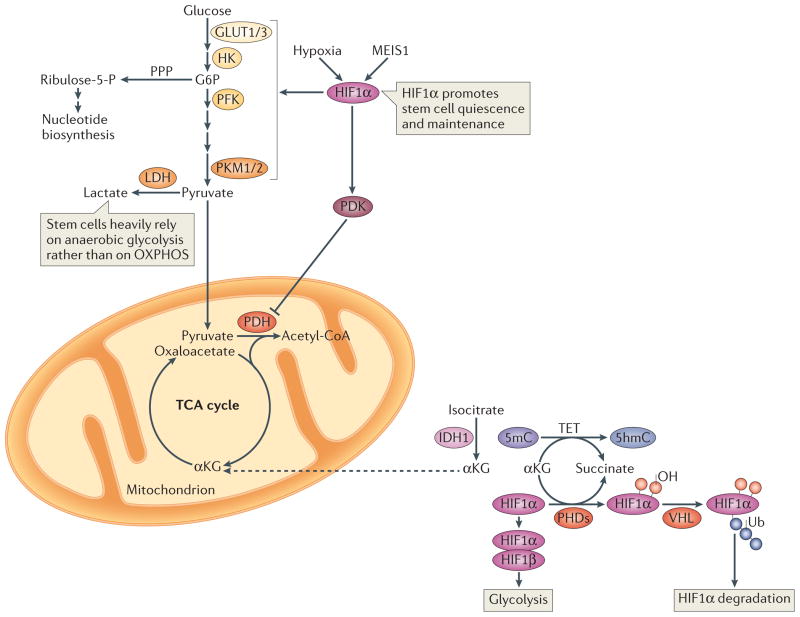
Figure 2. Glycolysis and hypoxia-inducible factor 1α.
Hypoxic conditions and myeloid ecotropic viral integration site 1 (MEIS1) activate hypoxia-inducible factor 1α (HIF1α), which promotes glycolysis and HIF1α-dependent pyruvate dehydrogenase kinase (PDK) activation. PDK in turn prevents pyruvate oxidation by suppressing the pyruvate dehydrogenase (PDH) complex. Such hypoxic conditions are crucial for stem cell maintenance. In normoxic conditions, prolyl hydroxylases (PHDs) catalyse HIF1α hydroxylation in the presence of iron and α-ketoglutarate (αKG), and generate succinate in the process of hydroxylating Pro residues in HIF1α. Hydroxylated HIF1α is targeted for degradation by the von Hippel–Lindau (VHL) ubiquitin ligase complex. A group of αKG-dependent dioxygenases, the ten-eleven translocation (TET) proteins, catalyze the oxidation of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) in the genome. TET proteins require iron for catalysis and oxidize prime substrates (such as 5mC into 5hmC) with coupled oxidation of αKG (the co-substrate) into succinate and CO2. TET deficiency in mice leads to skewed differentiation and enhanced repopulating capacity of haematopoietic stem cells (HSCs). Isocitrate dehydrogenase 1 (IDH1) catalyzes the oxidative decarboxylation of isocitrate to αKG and CO2. Dashed arrows indicate transport between mitochondria and the cytosol. G6P, glucose-6-phosphate; GLUT, glucose transporter; HK, hexokinase; LDH, lactate dehydrogenase; PFK, phosphofructokinase; PKM, pyruvate kinase muscle isozyme; PPP, pentose phospate pathway; OXPHOS, oxidative phosphorylation; TCA cycle, tricarboxylic acid cycle.