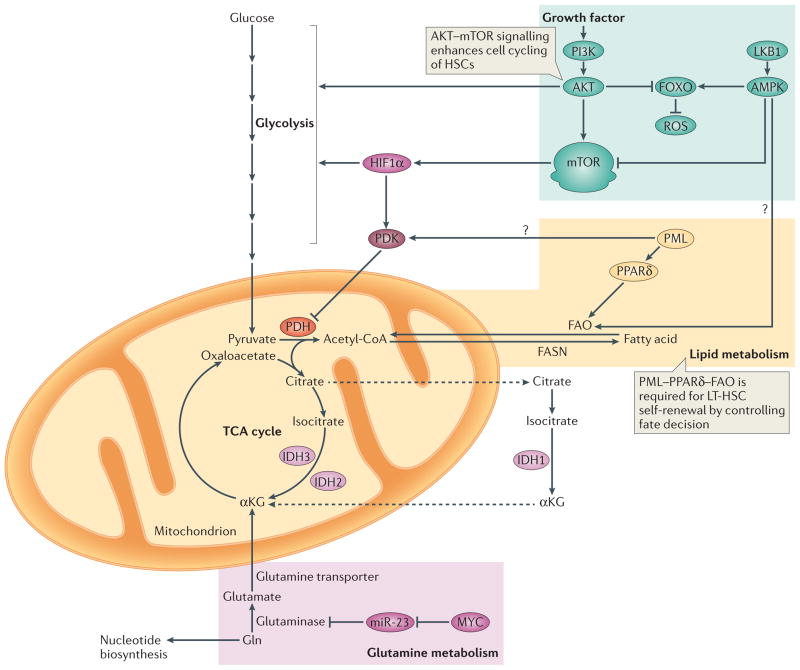
Figure 3. Overview of metabolic requirements in stem cells.
PI3K–AKT signalling promotes the production of reactive oxygen species (ROS) by repressing the forkhead box O (FOXO)-mediated stress response. This is accompanied by increased glycolysis and activation of mTOR, which promotes cell cycling of haematopoietic stem cells (HSCs) (indicated by green background). MYC controls the balance between HSC self-renewal and differentiation183,184 (indicated by pink background). Gln is converted to glutamate by glutaminase, which is under the control of MYC, in part through microRNA-23 (miR-23), and its role in stem cell homeostasis is currently being elucidated. The promyelocytic leukaemia protein (PML)–peroxisome proliferator activator receptor-δ (PPARδ) pathway for fatty acid oxidation (FAO) is required for long-term haematopoietic stem cell (LT-HSC) self-renewal and quiescence (indicated by orange background). Isocitrate dehydrogenases (IDHs) are a family of enzymes that are responsible for catalyzing the oxidative decarboxylation of isocitrate into α-chetoglutarate (αKG). IDH1 functions in the cytosol and peroxisomes, whereas IDH2 and IDH3 are both localized in the mitochondria. Dashed arrows indicate transport between mitochondria and the cytosol. Question marks indicate interesting links between metabolic programmes, although these connections have not been formally tested in stem cells. Acetyl-CoA, acetyl-coenzyme A; FASN, fatty acid synthase; LKB1, liver kinase B1; PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinase; TCA cycle, tricarboxylic acid cycle.