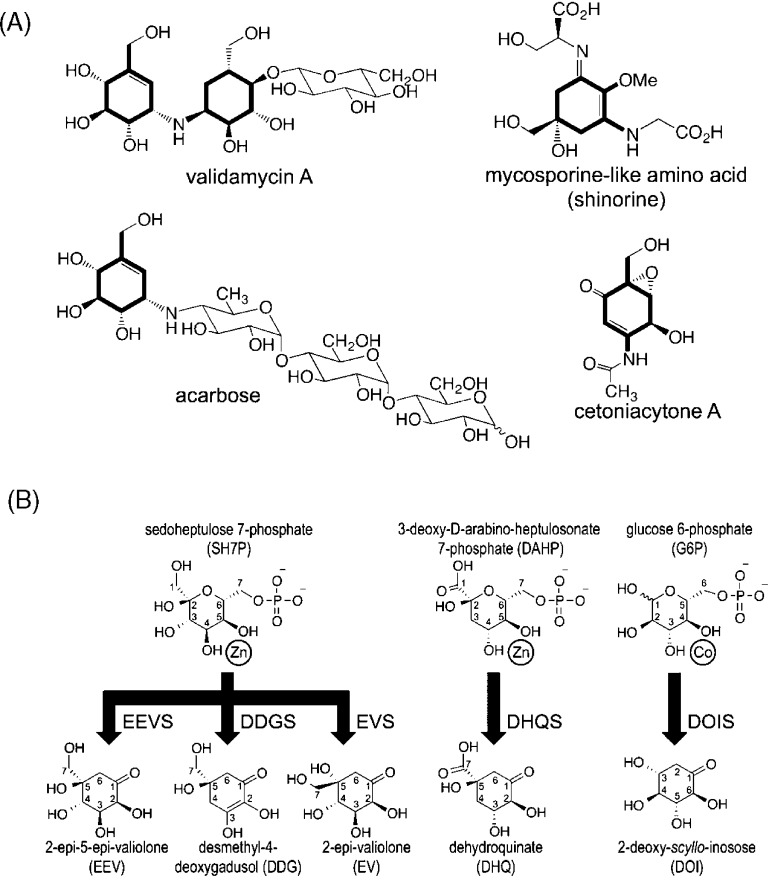
Figure 1.
Reactions catalyzed by known sugar phosphate cyclases. (A) Four cyclitol-containing natural products are shown and labeled by name, with their C7-cyclitol units made by SH7PCs highlighted in bold. (B) The substrates (above) and products (below) of five sugar phosphate cyclases are shown. A divalent metal cation is drawn next to the two substrate hydroxyls seen (for DHQS and DOIS) to coordinate it. SH7PCs and DHQS may utilize Zn2+ or Co2+ as the divalent metal cation6,40 but are shown here with Zn2+, the metal present in this structure of ValA and in structures of AnDHQS. DOIS uses only Co2+ for catalysis41 and is depicted as such. Abbreviations for the substrates and products are introduced, with each enzyme abbreviation being that of its product followed by an additional S for “synthase”. Because of resonance, DDG has an internal symmetry so the stereoconfiguration at C5 of the product after it is released into solution is not uniquely defined. We draw it here with the same stereochemistry as EEV, anticipating the proposal we make in this work that the cyclization products of EEVS and DDGS have the same C5 configuration.