Figure 7.
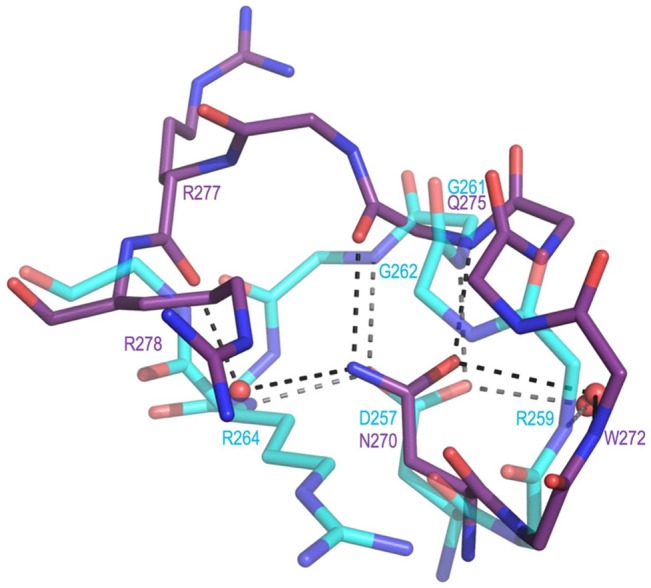
Active site loop difference that relates to the presence of Asp257 in DHQS vs Asn270 in ValA. Shown are residues 269–278 of ValA (purple) and residues 256–264 of DHQS (cyan, PDB entry 1DQS) after the proteins have been overlaid as in Figure 5. H-Bonding interactions (dashed lines) involving the loop residues and associated waters are shown. In DHQS, the Asp257 carboxylate receives H-bonds directly or indirectly (via water) from four backbone nitrogens (from Arg259, Gly261, Gly262, and Arg264). In ValA, the Asn270 side chain amide directly or indirectly makes H-bonds with two backbone nitrogens (from Trp272 and Glu275) and two backbone oxygens (from Gln275 and Arg277).
