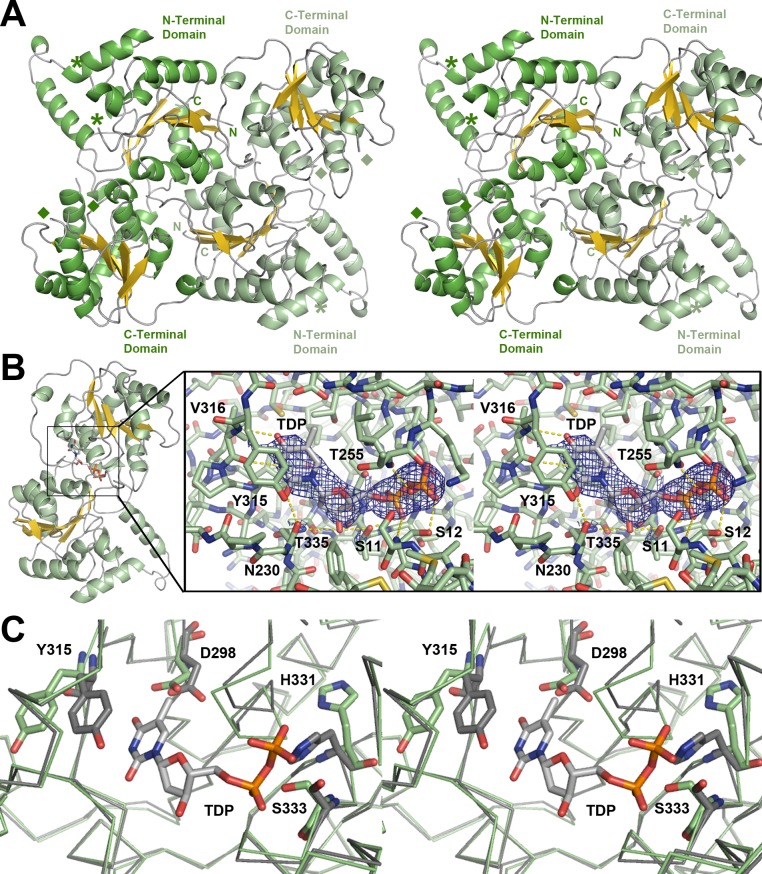
Figure 2.
SpnP homodimer. (A) Stereodiagram showing the asymmetric unit. Asterisks mark the beginning and end of the first disordered loop (FL1, residues 60–98). Diamonds mark the beginning and end of the second disordered loop (FL2, residues 258–277). (B) Stereodiagram of the donor substrate-binding site in the SpnP–TDP complex showing the Fo – Fc omit map (contoured at 2.5 root-mean-square deviations). The thymine base, deoxyribose, and the pyrophosphate of TDP contact the labeled residues in the donor-binding site. (C) Stereodiagram showing the SpnP active site in the absence of TDP (green) and in the presence of TDP (gray). The TDP α-phosphate stabilizes one conformation of the pyrophosphate-binding loop (H-X3-G-T motif), causing several residues to shift, notably H331 and S333. Y315 also reorients to stack with the thymine base.