Figure 5.
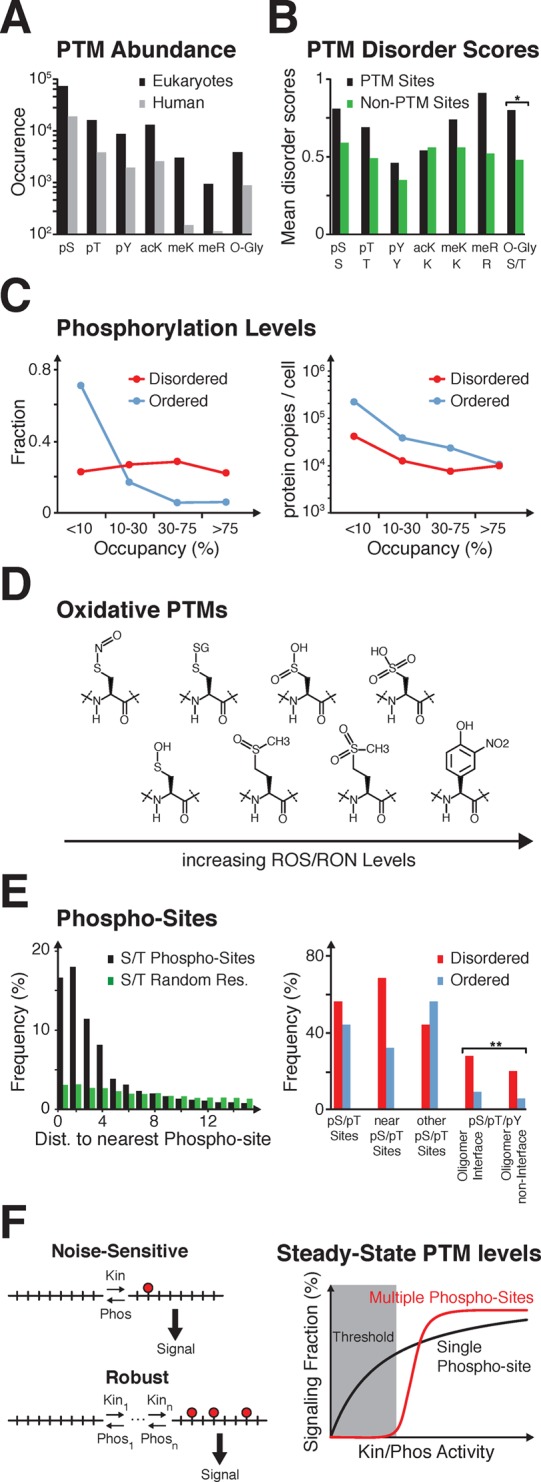
Post-translational modifications. (A) Experimental and predicted phospho-serines (pS), -threonines (pT), -tyrosines (pY), acetyl-lysines (acK), mono-, di-, or trimethyl-lysines (meK), symmetric and asymmetric dimethyl-arginines (meR), and O-glycosylated serines or threonines (O-Gly) according to ref (372). (B) Disorder scores for pS, pT, pY, acK, meK, and meR (black), and nonmodified residues (green), according to VSL2B, ref (382) and SwissProt, ref (380). Scores above or below 0.5 predict disorder or order, respectively. Scores for O-Gly (*) were calculated as the ratio of O-glycosylation sites in predicted IDRs over the total number of O-glycosylation sites in 190 proteins. For nonmodified sites, the ratio of serines/threonines in IDRs was determined over their numbers in the same set of proteins, ref (379). (C) Left: Fraction of pS/pT sites in disordered and ordered S. cerevisiae proteins and their relative phosphorylation levels (occupancy), according to ref (386). Right: Average number of proteins per S. cerevisiae cell and their relative phosphorylation levels, according to ref (394). (D) Chemical structures of post-translational amino acid modifications at increasing ROS/RNS levels. (E) Left: Distances between phosphorylated serine/threonine residues in eukaryotic proteins (black), versus average distances between random (modified and nonmodified) serine and threonine residues (green). Adapted with permission from ref (470). Copyright 2010 Biomed Central Ltd., Springer Science+Business media Right: Disorder and order at serine/threonine phosphorylation sites (pS/pT), at pS/pT sites with neighboring modification sites less than 4 residues apart (near pS/pT sites) and at pS/pT sites with no other modification site less than 4 residues away (other pS/pT sites). Adapted with permission from ref (470). Copyright 2010 Biomed Central Ltd., Springer Science+Business media. Average serine/threonine/tyrosine phosphorylation sites at oligomer interfaces and noninterface regions (**). Adapted with permission from ref (469). Copyright 2013 Royal Society of Chemistry. (F) Left: Sensitivity and robustness of single-, versus multiple-PTM signaling modes. Right: Switch-like responses result from multiple modifications in the presence of balanced kinase (Kin) and phosphatase (Phos) activities.
