Scheme 2.
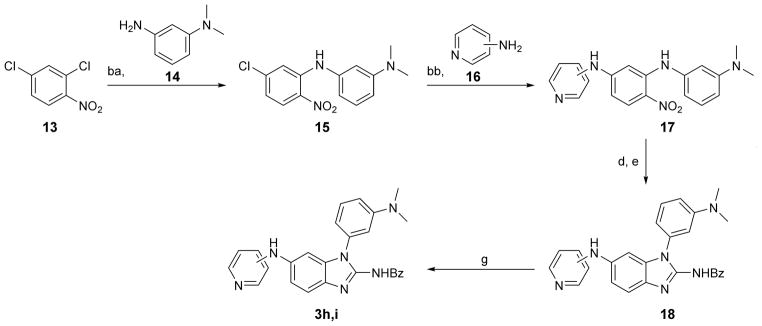
Synthesis of 1-aryl-2-aminobenzimidazoles 3h and 3i. Reagents and conditions: ba) Cs2CO3, Pd2(dba)3, (±)BINAP, toluene, reflux, 21 h, 43 % yield, two steps; 14 was synthesized from N,N-dimethyl-3-nitroaniline by reduction with H2 (1 atm), Pd/C in MeOH, 20 hr; bb) Cs2CO3, Pd2(dba)3, (±)BINAP, dioxane, reflux, 20–44 h, 34–43 % yield d) H2 (1 atm), Pd/C or PtO2, MeOH, RT, 21–23 h h; e) benzoylNCS, DIPC, DIPEA, ACN, 24–26 h, 22–32 % yield over two steps; g) 1N HCl, H2O, dioxane, reflux, 23–26 h, 27–56 % yield.
