Scheme 3.
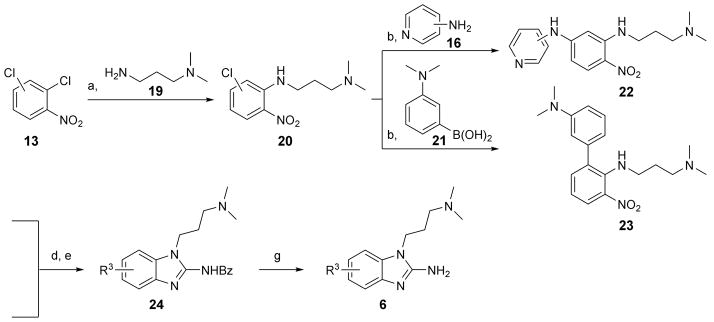
Synthesis of N,N-dimethylaminopropyl-substituted benzimidazoles 6 from dichloro-nitrobenzenes 13. Preparation of 6-substituted benzimidazoles commenced from 1,2-dichloro-4-nitrobenzene, that of 7-substituted products from 1,2-dichloro-3-nitrobenzene. Reagents and conditions: a) K2CO3, ACN, reflux, 24 h, 96–97 % yield; b) Cs2CO3, Pd2(dba)3, (±)BINAP, 19–23 h, 37–80 % yield range; d) H2 (1 atm), Pd/C or PtO2, MeOH, RT, 21–25 h; e) benzoylNCS, DIPC, DIPEA, ACN, 19–21 h, 29–33 % yield over two steps; g) 1N HCl, H2O, dioxane, reflux, 17–21 h, 22–46 % yield.
