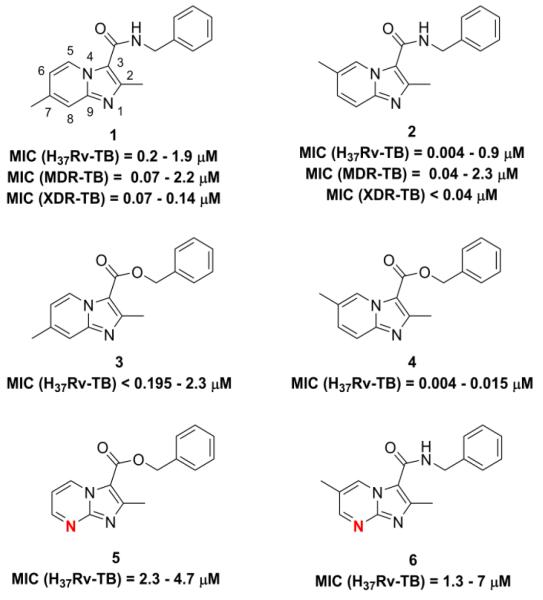
Figure 1.
Previously reported imidazo[1,2-a]pyridines (1-3) and imidazo[1,2-a]pyrimidine (5) and two new analogs prepared for this SAR study the benzyl 2,6-dimethylimidazo[1,2-a]pyridine-3-carboxylate (4) and the N-benzyl-2,6-dimethylimidazo[1,2-a]pyrimidine-3-carboxamide (6). The imidazo[1,2-a]pyrimidine nitrogen is denoted in red.