Scheme 1.
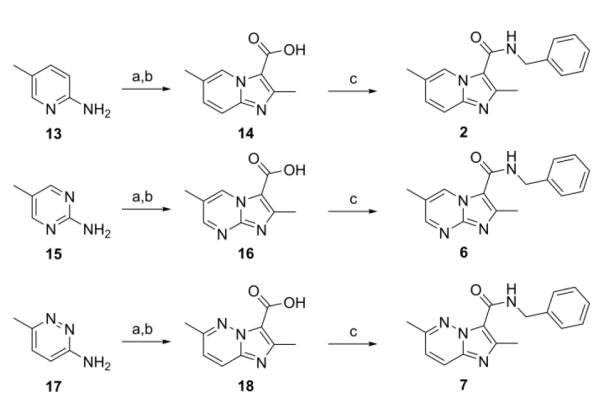
Synthesis of imidazo[1,2-a]pyridine (2), imidazo[1,2-a]pyrimidine (6), and imidazo[1,2-b]pyridazine (7). Reagents: (a) 1. Ethyl 2-chloroacetoacetate (for 13) or Ethyl 2-bromoacetoacetate (for 15 and 17), DME, reflux, 48 h.; (b) 1. LiOH, EtOH; 2. HCl, 56 h.; (c) EDC, DMAP, benzyl amine, ACN, 16 h.
