Abstract
Action representations associated with object use may be incidentally activated during visual object processing, and the time course of such activations may be influenced by lexical-semantic context (e.g., Lee, Middleton, Mirman, Kalénine, & Buxbaum, 2012). In this study we used the “visual world” eye-tracking paradigm to examine whether a deficit in producing skilled object-use actions (apraxia) is associated with abnormalities in incidental activation of action information, and assessed the neuroanatomical substrates of any such deficits. Twenty left hemisphere stroke patients, ten of whom were apraxic, performed a task requiring identification of a named object in a visual display containing manipulation-related and unrelated distractor objects. Manipulation relationships among objects were not relevant to the identification task. Objects were cued with neutral (“S/he saw the….”), or action-relevant (“S/he used the….”) sentences. Non-apraxic participants looked at use-related non-target objects significantly more than at unrelated non-target objects when cued both by neutral and action-relevant sentences, indicating that action information is incidentally activated. In contrast, apraxic participants showed delayed activation of manipulation-based action information during object identification when cued by neutral sentences. The magnitude of delayed activation in the neutral sentence condition was reliably predicted by lower scores on a test of gesture production to viewed objects, as well as by lesion loci in the inferior parietal and posterior temporal lobes. However, when cued by a sentence containing an action verb, apraxic participants showed fixation patterns that were statistically indistinguishable from non-apraxic controls. In support of grounded theories of cognition, these results suggest that apraxia and temporal-parietal lesions may be associated with abnormalities in incidental activation of action information from objects. Further, they suggest that the previously-observed facilitative role of action verbs in the retrieval of object-related action information extends to participants with apraxia.
Keywords: object use, apraxia, eye tracking, parietal, temporal
1. Introduction
1.1. Apraxia
Limb apraxia (hereafter, simply “apraxia”) is a disorder of complex skilled action not attributable to weakness, incoordination, or other elemental sensory or motor impairments. It occurs in approximately 50% of people who have suffered left hemisphere cerebral vascular accidents (LCVA) (Barbieri & De Renzi, 1988; Zwinkels, Geusgens, Sande, & Heugten, 2004). Classic accounts distinguish two major subtypes of apraxia, termed ideational and ideomotor. Ideomotor apraxia is frequently assumed to affect the accuracy of gesture pantomime and imitation due to abnormalities in joint angles and limb trajectories, and uncoupling of the spatial and temporal aspects of movement (Haaland, Harrington, & Knight, 1999; Smania et al., 2006; Smania, Girardi, Domenicali, Lora, & Aglioti, 2000). Ideational apraxia is traditionally distinguished on the basis of tool misuse errors on single and multiple-objects tasks (see Vanbellingen & Bohlhalter, 2011, for review). However, in practice, these distinctions have been difficult to validate. Deficits on pantomime tasks and impairments in real object use have been shown to correlate significantly in a positive direction (Randerath, Li, Goldenberg, & Hermsdörfer, 2009); furthermore, individuals with apraxia make similar types of errors on both tasks (Clark et al., 1994; Mcdonald, Tate, & Rigby, 1994; Poizner et al., 1995).
A long history in the apraxia literature attributes object misuse errors to impaired “action semantics”, specifically, impaired knowledge of the manner in which particular objects are manipulated (De Renzi & Lucchelli, 1994; Heilman, Rothi, & Valenstein, 1982; Morlaas, 1928; Stamenova, Roy, & Black, 2010, see Bouillaud, 1825; Lordat, 1843, for similar proposals for articulation in aphemia). This comports with accounts of conceptual knowledge proposing that conceptual information is distributed across the same network of sensory and motor attribute domains activated when the information was first acquired (Allport, 1985; Barsalou, 2008; Stamenova et al., 2010; Warrington & McCarthy, 1987; Warrington & Shallice, 1984; see also Damasio, 1990; Shallice, 1988, but see Garcea, Dombovy, & Mahon, 2013 and Mizelle & Wheaton, 2010 for different views). In fact, consistent with a conceptual deficit, many stroke patients with apraxia are deficient in the recognition of skilled hand-object interactions and object-related hand postures (Buxbaum, Johnson-Frey, & Bartlett-Williams, 2005; Buxbaum, Kyle, Grossman, & Coslett, 2007; Buxbaum, Sirigu, Schwartz, & Klatzky, 2003; Buxbaum, 2001; Sirigu et al., 1995, 1996), and have difficulty learning new object-related gestures (Faglioni, Basso, Botti, Aglioti, & Saetti, 1990; Rothi & Heilman, 1985). For example, it has been shown that apraxics have difficulty matching familiar objects with the hand postures appropriate for their use (Buxbaum et al., 2003), or matching objects based on the similarity of their associated functional actions (Buxbaum & Coslett, 1998). In contrast, apraxics perform normally in producing or recognizing hand postures appropriate for grasping objects based on their structural properties (shape and size) (Buxbaum et al., 2003).
Object use and object pantomime deficits typically occur after lesions to left inferior parietal cortex (IPL) (Buxbaum et al., 2007, 2003; Haaland, Harrington, & Knight, 2000; Heilman et al., 1982; Randerath, Goldenberg, Spijkers, Li, & Hermsdörfer, 2010), although apraxia has also been observed after lesions in premotor areas, including middle frontal and inferior frontal gyri (Goldenberg, 2009; Haaland et al., 2000; Heilman et al., 1982). Lesions to left IPL as well as the posterior middle temporal gyrus (pMTG) also impair the recognition of familiar object use actions (Kalénine, Buxbaum, & Coslett, 2010). These regions overlap those that are activated in functional neuroimaging studies of manipulation knowledge (e.g., Boronat et al., 2005; Kalénine et al., 2010; Kellenbach, Brett, & Patterson, 2003) and object-related movements (see Caspers, Zilles, Laird, & Eickhoff, 2010 and Watson, Cardillo et al., 2013 for meta-analyses).
A relatively understudied issue concerns the mechanisms underlying impaired action knowledge in apraxics. It has been shown that apraxic patients’ knowledge of object-associated use-actions (i.e., manipulation knowledge) may be selectively impaired despite preservation of knowledge of objects’ functional purpose (Buxbaum, Veramonti, & Schwartz, 2000; Buxbaum & Saffran, 2002). However, based on prior findings, it is not clear whether use-action knowledge in apraxics is entirely degraded, or whether it is relatively intact but difficult for apraxics to access. Similar distinctions between representational access and integrity have been investigated in a range of brain-damaged patients, including those with blindsight (e.g., Poppel, Held, & Frost, 1973), hemispatial neglect (e.g., Marshall & Halligan, 1988), aphasia (e.g., Blumstein, Milberg, & Shrier, 1982; Mirman & Britt, 2014), dyslexia (Colangelo, Stephenson, et al, 2003) and semantic deficits (e.g., Campanella, Mondani, et al., 2009; Predovan, Gandini, et al., 2013; Reilly, Peelle, et al., 2011). In a number of these cases, impaired performance in explicit behavioral tasks is accompanied by relatively intact performance when assessed implicitly. For example, the performance of patients with neglect or extinction may be influenced by visual stimuli despite lack of conscious detection (e.g., Ladavas, Paladini, & Cubelli, 1993; di Pellegrino, Rafal, & Tipper, 2005; Rafal, Ward, & Danziger, 2006; Riddoch, Riveros, & Humphreys, 2011), and Wernicke’s aphasics’ lexical processing can be primed by a semantically related word despite their poor performance in explicit semantic relatedness judgment tasks (Blumstein et al., 1982; Milberg & Blumstein, 1981). These findings suggest that impairment in overt behavioral responses may not reliably assess the status of conceptual knowledge.
The integrity and accessibility of conceptual knowledge in stroke patients has also been interpreted on the basis of their performance in conditions of priming or cuing (e.g., Auchterlonie, Phillips, & Chertkow, 2002; Brambati, Peters, Belleville, & Joubert, 2012; Corbett, Jefferies, & Lambon Ralph, 2011; Jefferies, Baker, Doran, & Ralph, 2007; Jefferies, Patterson, & Lambon Ralph, 2008; Jefferies & Lambon Ralph, 2006; Tyler & Ostrin, 1994; Warrington & Shallice, 1979). In such cases, improvement with increased ‘retrieval cues’ is often held to indicate that conceptual representations are relatively intact but poorly accessible, and absence of cueing effects are taken to indicate the representations are completely lost (Corbett et al., 2011; Jefferies et al., 2007, 2008; Jefferies & Lambon Ralph, 2006). As will be described below, we can make use of such hypothesized distinctions to shed light on the nature of the action knowledge deficit in apraxia. First, however, we will review relevant studies with healthy participants demonstrating incidental activation of action information during object processing and modulation of such activations by retrieval cues.
1.2. Action influences object identification in healthy participants
Several studies with healthy participants have shown that manual action information may be accessed during object processing even when action is entirely incidental to task demands. Strong evidence for this claim comes from studies using the “Visual World Paradigm” (VWP), a paradigm widely used in healthy participants (Huettig & Altmann, 2005, 2007) as well as patient populations (Kalénine, Mirman, & Buxbaum, 2012; Mirman & Graziano, 2012; Mirman, Yee, Blumstein, & Magnuson, 2011; Myung et al., 2010; Yee, Blumstein, & Sedivy, 2007). In a typical VWP study, participants’ eye movements are recorded while they point to or click on an auditorially-cued target picture shown as part of a visual display. A related distractor (“competitor”) that shares attributes of interest with the target is typically also displayed, along with unrelated distractor pictures that do not share these attributes (e.g., Huettig & Altmann, 2005; Mirman & Magnuson, 2009; Yee & Sedivy, 2006). For example, for a given target object such as ‘typewriter’, the distractors might include an object sharing action attributes with the target (the related distractor, e.g., ‘piano’) as well as objects unrelated to the target in action (the unrelated distractor, e.g., ‘couch’; examples taken from Myung, Blumstein, & Sedivy, 2006). With such paradigms, it has been shown that participants tend to fixate more on distractors similar to the targets in manipulation actions than on unrelated distractors (Lee, Middleton, Mirman, Kalénine, & Buxbaum, 2012; Myung, Blumstein, & Sedivy, 2006). As the related and the unrelated distractors in the same display are typically matched on other critical features (e.g., visual complexity, familiarity, general semantic similarity, etc.), fixations on the related relative to unrelated distractors (the “competition effect”) can be used to infer incidental activation of action information.
Furthermore, it has been shown that incidental access to action information may be ‘primed’ or modulated by several types of cues. For example, in a recent VWP study (Lee et al., 2012), the activation time course of action information was modulated by provision of an action verb context (e.g., he “used the ______”), leading to an earlier-emerging competition effect or faster target detection. Furthermore, target identification is influenced by implicit action relationships between objects in blocked cyclic paradigms associated with a build-up of semantic interference (Campanella & Shallice, 2011a, 2011b) as well as during rapid presentation (Roberts & Humphreys, 2011) and in visual scenes requiring perceptual integration (Green & Hummel, 2006).
1.3. Incidental activation of use information in apraxic participants?
Recently, Myung and colleagues (2010) used the VWP to implicitly assess the activation time course of action information in 4 apraxic and 5 non-apraxic stroke patients. Like non-apraxic (Myung et al, 2010) and healthy participants (Myung et al. 2006), apraxics fixated more on action-related than unrelated distractors during target identification. However, they were slower to look at action-related distractors than were non-apraxics. These results were taken to suggest that apraxics are deficient in accessing manipulation features of objects.
However, there are several limitations of the study of Myung et al. (2010). First, many of the related distractor objects shared grasp actions as well as use actions with the targets. For example, both the target ‘Paper Clip’ and the related distractor ‘Clothespin’ may be picked up as well as functionally used with a pinch hand posture. Apraxics are typically relatively unimpaired in producing grasp actions (e.g., Buxbaum & Kalénine, 2010, but see Randerath, Goldenberg, Spijkers, Li, & Hermsdörfer, 2010 for findings showing apraxic patients can be more error-prone in grasping when attempting to subsequently use a tool); thus, the fact that action-related competition effects were present (albeit delayed) in apraxics may have been driven by the integrity of representations subserving grasping (see Jax & Buxbaum, 2013). In addition, in the study of Myung et al. (2010) the association of apraxia severity and delayed onset of the competition effects was suggestive, but not statistically significant (p=.11), making it difficult to attribute the observed abnormalities in competition effects to the apraxia, per se. Finally, subjects’ lesion information was unavailable, making it difficult to exclude the possibility that the delayed competition effects may have been influenced by lesion volume and thus, overall severity of brain damage.
2. The present study
In view of the outstanding issues, the current study was designed to extend the findings of Myung et al. (2010) with more refined stimuli, a bigger sample size, and relevant anatomical and neuropsychological information. Specifically, we used the VWP design previously tested with healthy participants (Lee et al., 2012) in which target objects shared only use but not grasp actions with related distractor objects. For example, one such target was a television remote control, which is picked up and moved with a clench posture, but used with a poke (we call such objects “conflict” objects because of the conflicting actions associated with them). The critical related distractor object in this case was a car key fob, which is picked up and moved with a pinch, but, like the television remote, is used with a poke. With this design, increased gaze fixations on related distractors as compared to unrelated distractors can be unambiguously attributed to incidental activation of use information during target recognition because the target and related distractors share the use action only and not the grasp action. To provide an additional assessment of whether apraxics’ impairment with use-action information is due to deficits in representational access or integrity, we further manipulated the types of context provided. Specifically, we compared performance in two context conditions: one including an action verb associated with limb actions (‘S/he used the ....’), and the other containing only a neutral verb (‘S/he saw the….’). As in previous studies, participants’ task was to identify the picture that matched an auditory target word by clicking on the picture with a computer mouse (i.e., spoken word-to-picture matching).
By comparing eye fixations on a function-based related distractor with fixations on unrelated distractors, we assessed the time course and degree of activation of function-based use-action information, and compared it across the two contexts and across apraxic and non-apraxic control participants. If apraxics suffer from substantially degraded use-action knowledge, we expect a substantial reduction in or absence of competition effects, that is, no significant difference between fixation patterns on related versus unrelated distractors, regardless of context type. If apraxics’ behavioral deficits are instead mainly due to deficits in access to use-action knowledge, we expect to see relatively preserved but perhaps later-emerging competition effects. Moreover, if use knowledge is relatively intact but difficult to access, we should observe facilitation by an action verb context such as has been demonstrated in healthy participants (Lee et al., 2012).
Finally, we incorporated lesion information in the analysis of eye tracking data. The lesion information collected from the stroke patients provides a basis for partialling out the influence of overall severity of brain injury on the eye movement patterns, which was not done in the previous study (Myung et al., 2010). We also conducted exploratory lesion analyses to identify brain regions potentially associated with abnormalities in competition from function-based related distractors. Based on prior findings (e.g., Barde, Buxbaum, & Moll, 2007), we expected lesions in the inferior parietal lobe (angular gyrus [AG] and/or supramarginal gyrus [SMG]) to be associated with reduced and/or slowed function-based competition effects. However, given that inferior and middle frontal regions have been associated with skilled action production (e.g., Caspers, Zilles, et al., 2010; Haaland & Flaherty, 1984), and pMTG has been associated with object-related action knowledge (e.g., Kalénine et al., 2010), we also considered the role of these regions in any observed fixation abnormalities.
3. Methods
3.1. Participants
Twenty post-acute left hemispheric stroke patients participated in this study (12 males). All participants had unilateral cortical lesions and were right handed prior to stroke. All had normal or corrected-to-normal vision and were permitted to wear glasses during the eye tracking session if needed. Participants were recruited from the Moss Rehabilitation Research Institute research registry (Schwartz, Brecher, Whyte, & Klein, 2005). Participants over the age of 80 years and/or with histories of co-morbid or pre-morbid neurologic disorders, alcohol or drug abuse, or psychosis were excluded, as were participants whose records indicated severe comprehension deficits. All participants gave informed consent to participate in accordance with the IRB guidelines of the Einstein Healthcare Network and the University of Pennsylvania School of Medicine, and were paid for their participation.
Neuropsychological data
To assess general language comprehension, participants performed the comprehension sub-test of the Western Aphasia Battery (WAB; Kertesz, 1982), which assesses responses to yes/no questions, sequential commands, and word-object matching. Participants also completed a test of ability to produce pantomimed gestures to the sight of real objects (i.e., without picking up the objects) with the less affected left hand (Buxbaum, Veramonti, & Schwartz, 2000) and a gesture recognition test (Buxbaum, Kyle, & Menon, 2005; Kalénine et al., 2010). Trials on the ‘gesture to sight’ task were scored correct or incorrect on four movement components (hand posture, arm posture, amplitude, and timing) by trained, reliable coders who were blind to the study hypotheses (see Buxbaum et al., 2000, for details of the scoring criteria and reliability information). In the gesture recognition test, which is comprised of Semantic Gesture Recognition and Spatial Gesture Recognition subtests, an action word (e.g., ‘‘hammering’’) was presented on a computer screen and concurrently read aloud by the experimenter, followed by a video of a person making 2 different gestures sequentially. Participants’ task was to verbally indicate the gesture that correctly illustrated the action. In the Semantic Gesture Recognition task, foil gestures were incorrect by virtue of a semantic relationship with the target (e.g., Target: combing hair; foil: brushing teeth). In the Spatial Gesture Recognition task, foils included a spatial modification in hand, arm, or amplitude/timing components (e.g., Target: sawing; foil: correct arm movement and posture with an incorrect ‘‘clawed’’, splay-fingered hand posture). (see Kalénine et al., 2010 for additional detail). Results are provided in Table 1.
Table 1.
Individual demographic information, lesion volume, and neuropsychological scores for apraxic and non-apraxic patients. Group averages are provided at the bottom of each group; standard deviations are in parentheses.
| Group | No. | Age (yrs) | Gender | Edu. (yrs) | Time post- stroke | Lesion Volume (# damaged voxels) | WAB Comp. (out of 10) | Gesture- to-sight (%) | Gesture recognition (%) |
|---|---|---|---|---|---|---|---|---|---|
| Non- apraxic | 1 | 58 | M | 16 | 11Y6M | 103943 | 8.5 | 82.5 | 88.85 |
| 2 | 43 | F | 12 | 8Y11M | 51860 | 9.2 | 92.5 | 93.5 | |
| 3 | 48 | F | 18 | 5Y8M | 89072 | 9.5 | 90.0 | 93.4 | |
| 4 | 53 | M | 13 | 6Y1M | 172209 | 9.9 | 97.5 | 95.65 | |
| 5 | 67 | M | 19 | 4Y1M | 84923 | 9.4 | 93.0 | 97.9 | |
| 6 | 74 | M | 12 | 4Y0M | 77326 | 9.8 | 95.0 | 91.35 | |
| 7 | 73 | M | 20 | 3Y5M | 40953 | 8.9 | 95.0 | 91.65 | |
| 8 | 54 | M | 12 | 3Y9M | 57638 | 8.7 | 92.5 | 81.8 | |
| 9 | 62 | F | 14 | 2Y10M | 51525 | 10.0 | 90.0 | 95.8 | |
| 10 | 59 | M | 15 | 4Y3M | 195293 | 9.0 | 80.0 | 83.9 | |
| Avg (sd) | 59.1 (10.2) | -- | 15.1 (3.0) | 5Y5M (2Y9M) | 92474 (52232) | 9.3 (0.5) | 90.8 (5.6) | 91.38 (4.93) | |
| Apraxic | 1 | 57 | F | 17 | 11Y1M | 224410 | 7.0 | 65.0 | 86.85 |
| 2 | 51 | F | 16 | 11Y0M | 151318 | 9.9 | 77.5 | 80.95 | |
| 3 | 50 | M | 14 | 2Y8M | 5376 | 9.5 | 50.0 | 82.6 | |
| 4 | 66 | M | 16 | 3Y6M | 271984 | 4.8 | 50.0 | 71.6 | |
| 5 | 56 | M | 12 | 2Y8M | 25273 | 8.7 | 70.0 | 93.3 | |
| 6 | 61 | F | 16 | 1Y5M | 73100 | 9.6 | 72.5 | 93.5 | |
| 7 | 64 | F | 12 | 4Y2M | 110296 | 5.3 | 67.5 | 70.1 | |
| 8 | 46 | F | 12 | 3Y4M | 140554 | 9.2 | 45.0 | 80.95 | |
| 9 | 48 | M | 14 | 2Y5M | 55685 | 8.5 | 65.0 | 93.3 | |
| 10 | 79 | F | 18 | 6Y11M | - | 9.2 | 70.0 | 83.55 | |
| Avg (sd) | 57.8 (10.1) | -- | 14.7 (2.2) | 4Y11M (3Y6M) | 117555 (89367) | 8.2 (1.9) | 63.3 (11) | 83.7 (7.99) |
Participant classification
Participants were characterized as apraxic1 if their score on the gesture-to-sight of objects test fell below a normative cutoff (<79.9% correct) based on performance 2 standard deviations below the mean of healthy subjects (Buxbaum et al., 2005; Jax, Buxbaum, & Moll, 2006; Kalénine et al., 2010) (mean score=90.9, SD=5.5). Ten of the participants scored below the cutoff. Note that in addition to classifying patients as apraxic or not, gesture-to-sight scores were also used as a continuous variable in the analyses reported below. Gesture recognition scores were not used to classify subjects; however, gesture recognition scores were strongly correlated with gesture-to-sight scores (r(18)=.61, p<.01), consistent with the results of previous investigations (Buxbaum, Kyle, & Menon, 2005). Two-tailed t tests confirmed that the designated apraxics performed reliably more poorly than did designated non-apraxics on both gesture-to-sight (t(18)=7.1, p<.001) and gesture recognition (t(18)=2.5, p<.05) tests.
Across groups, apraxics and non-apraxics were matched for age, education level, WAB comprehension score, and total lesion volume (p = .8 for age and education, p values ≥ 0.1 for WAB and total lesion volume). Nevertheless, there was a numerical trend toward greater severity of apraxics in WAB comprehension scores and total lesion volume. In order to control for these influences, WAB comprehension and Total Lesion Volume were included in all subsequent analyses as covariates. Demographic, lesion, and apraxia test data are provided in Table 1.
Imaging data
Structural MRI or CT scans were acquired for all participants. Twelve participants received MRI scans; nine were high-resolution whole-brain T1-weighted images (repetition time = 1620 ms, echo time = 3.87 ms, field of view = 192 x 256 mm, 1 x 1 x 1mm voxels) acquired with a Siemens 8-channel head coil on a 3T Siemens Trio scanner. Due to contraindications for a 3T environment, three subjects received whole-brain T1-weighted images acquired on a 1.5 T Siemens Sonata with a slice thickness of 1mm using a standard radio-frequency head coil (repetition time = 3000 ms, echo time = 3.54, field of view = 24 cm). Eight participants underwent whole-brain CT scans without contrast (60 axial slices, 3–5mm slice thickness) on a 64-slice Siemens SOMATOM Sensation scanner due to contraindications for MRI. The distributions of imaging types are closely matched across apraxia designation (non-apraxic: 2 1.5T-MRI, 4 3T-MRI, 4 CT; apraxic: 1 1.5T-MRI, 5 3T-MRI, 4 CT. One apraxic’s lesion could not be drawn due to a poor quality CT scan).
For the participants who had received MRI scans, lesions were segmented manually on a 1 x 1 x 1mm T1-weighted structural image. The structural scans were registered to a common template using a symmetric diffeomorphic registration algorithm (Avants, Schoenemann, & Gee, 2006; see also http://www.picsl.upenn.edu/ANTS/). This same mapping was then applied to the lesion maps. To optimize the automated registration, volumes were first registered to an intermediate template constructed from images acquired on the same scanner. A single mapping from this intermediate template to the Montreal Neurological Institute (MNI) space ‘Colin27’ volume (Holmes et al., 1998) was used to complete the mapping from subject space to MNI space. The final lesion map was quantized to produce a 0 or 1 map, with zeros indicating preserved voxels and ones indicating impaired voxels. After being warped to MNI space, the manually drawn depictions of the lesions were inspected by an experienced neurologist (Dr. H. Branch Coslett) who was naive with respect to the behavioral data.
For participants who had received CTs, lesion maps were drawn by Dr. Coslett directly onto the Colin27 volume, after rotating (pitch only) the template to approximate the slice plane of the participant’s scan with MRICron (http://www.cabiatl.com/mricro/mricro/index.html). For the rest of the participants, however, the individual lesions were visually inspected and analogue areas marked as lesioned on the template. Good intra- and inter-rater reliability has been previously demonstrated with this method (Schnur et al., 2009). The lesion coverage for the 19 participants is presented in Fig. 1.
Figure 1.
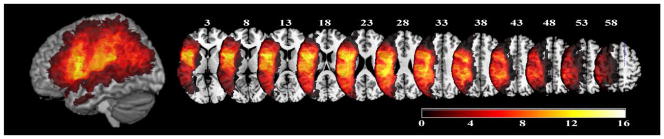
Lesion overlap of 19 participants whose structural MRI or CT scans were available. Color bar indicates the number of participants with a lesion in the region that is colored.
Two types of exploratory lesion analyses were performed. Whole-brain voxel based lesion symptom mapping was first carried out using VoxBo (www.voxbo.org) for identifying the voxels potentially relevant to later peaks of competition effects in the raw eyetracking data. Based on the results, a region of interest was defined. The association between this ROI and the overall time course of the competition effect was then assessed with Growth Curve Analysis. For each participant, the proportion of lesioned voxels within this ROI was used to predict the overall curve of the competition effect in the “SAW” context. Detailed analysis protocols are described in the Results section.
3.2. Materials
The materials were identical to those used by Lee et al. (2012), Experiment 2. In brief, the visual stimuli were arrays of four colored object images. Each array included a target, a related distractor, and two unrelated distractor images. All target images were conflict objects involving distinct skilled use and grasp-to-move action gestures (e.g., a TV remote control). Related distractors shared use, but not grasp action features with their corresponding targets (e.g., a keyfob). For unrelated items, care was taken to assure that no unrelated items shared any action features with the corresponding target or the related distractor. Related and unrelated distractors were matched for their visual similarity with the target based on rating scores from 16 healthy subjects. Mean rating scores were 3.1 and 2.9 for related and unrelated distractors respectively; 7 = highly similar, 1 = not at all similar; two-tailed paired-samples t-test: p = 0.8. Auditory stimuli included the target names (e.g., ‘the stapler’), two carrier phrases for action verb contexts (e.g., ‘She/He picked up’) and two for neutral contexts (e.g., ‘She/He saw’), all recorded by a female native speaker of English. Onset of the target words started 1400ms after the beginning of the sound file.
Twenty-two critical arrays of color images were presented once with an action verb context (‘s/he used the ….’) and once with a neutral context (‘s/he saw the…’) (see an example in Fig. 2). In addition, 88 arrays were created as filler trials from the 22 critical arrays according to the following scheme (also illustrated in Table 2). To make the target images in the critical trials unpredictable and reduce the prominence of the action verb context, each critical array was presented on a third trial in the neutral context, but with one of the original unrelated items as the target (e.g. Filler 1 in Table 2). To make the relation between the targets and competitors less noticeable and to again reduce the predictability of conflict objects being the targets, each of the distracter items in the original critical arrays was mixed with three other new images to form new arrays and served as targets in these new arrays (e.g. Filler 2–4 in Table 2). Among these new images, two were occurrences of a target from another critical array (e.g. Corkscrew in Filler 2 and 3 was the target image from another critical array). Half of these 66 new arrays (e.g. Filler 2–4 in Table 2) were presented with the neutral context and the other half with the action verb context. Overall, each participant saw 132 trials, of which 44 were critical trials. Of the 132 trials in total, 77 trials had a neutral verb context and 55 had an action verb context.
Figure 2.
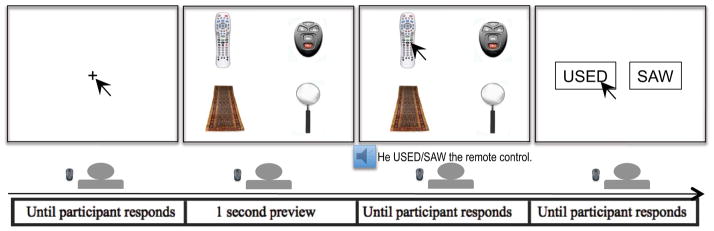
Procedure used in each trial. The display presents the target object (e.g., TV remote control), a related distractor (e.g., car key fob), and two unrelated distractors (e.g., rug and magnifying glass). The auditory stimuli starts after a 1,000ms preview of the display and the target word occurs at approximately 1400ms after the onset of the sentence. The location of target, related, and unrelated distracters was randomized on each trial. The arrows indicate the expected responses to the trial.
Table 2.
Examples for the scheme used for generating filler trials.
| Critical/ filler | Context | Target | Non-target | Non-target | Non-target |
|---|---|---|---|---|---|
| Critical | Neutral | Wire- cutters | Tongs (related) | Airplane (unrelated) | Brush (unrelated) |
| Critical | Action | Wire- cutters | Tongs (related) | Airplane (unrelated) | Brush (unrelated) |
|
| |||||
| Filler 1 | Neutral | Brush | Tongs | Wire-cutters | Airplane |
| Filler 2 | Action | Tongs | Nutcracker | Cheese-grater | Shell |
| Filler 3 | Neutral | Airplane | Sink | Cheese-grater | Mascara |
| Filler 4 | Action or neutral | Brush | Nutcracker | Cheese-grater | Shell |
3.3. Apparatus
Gaze position was recorded using an EyeLink 1000 remote (head free) desktop-mount eyetracker at 250 Hz after the standard nine-point calibration procedure. Eye movement data were parsed into fixations using the built-in algorithm with default settings. Stimulus presentations and response recording were conducted by E-Prime software (Psychological Software Tools, Pittsburgh, PA).
3.4. Procedure
The procedure was identical to Lee et al. (2012), Experiment 2 (illustrated here in Fig. 2). Participants were seated with their eyes approximately 27 inches from a 17-inch screen (resolution 1024 x 768 pixels). Each trial started with the participant clicking on a central fixation cross. Four images were presented simultaneously subsequent to the mouse click; each image, subtending approximately 3.5° of visual angle, was presented near one of the screen corners with a maximum size of 200 x 200 pixels. The location of target, related, and unrelated distracters was randomized on each trial. After a 1 second preview to allow for initial fixations driven by random factors or visual salience (as opposed to concept processing), participants heard the auditory stimuli through speakers. They were instructed to click on the image corresponding to the word at the end of the sentence as fast as possible. Upon the mouse-click response, the visual array disappeared and was replaced by two text boxes presented side-by-side on the screen, each containing one verb (‘saw’ or ‘used’). Participants were instructed to click on the verb mentioned in the sentence they just heard. This was to ensure that participants were paying attention to the sentence context during the experiment. The text boxes disappeared upon participants’ mouse-click response, terminating the trial. All participants responded with their left unimpaired hand. Prior to the experiment, participants were given a 30-trial practice session to orient them to the task.
3.5. Eye movement recording and data analysis
Eye movements were recorded from the beginning of each trial until the mouse-click response on the images. Four areas of interest (AOI) associated with the displayed pictures were defined as 400x300 pixel quadrants situated in the 4 corners of the computer screen. Fixations were counted toward each object type (Target, Related distractor, and Unrelated distractors) when falling into the corresponding AOI. Fixation proportions, the probabilities of participants looking at each AOI, were calculated for each time frame with the total number of trials as the denominator to avoid the selection bias introduced by varying trial-termination times (Mirman, Britt, & Cho, 2012). To reduce noise in the time course estimates of the fixations and to facilitate statistical model fitting (described in the next section), statistical analysis was conducted on the averages of every twenty 4ms frames (every 80ms time bin), a temporal resolution comparable to or better than in previous patient eyetracking studies (Kalénine et al., 2012; Myung et al., 2010; Yee et al., 2007). Trials for which participants failed to accurately click on the target pictures were excluded in subsequent analyses.
With fixation proportions in each time frame as our dependent measure, we aimed to analyze whether fixation proportion patterns over the time course of target identification differed between related and unrelated distractors as a function of context verb and/or patient group. To that end, Growth curve analysis (GCA) with orthogonal polynomials was used to quantify fixation differences across conditions during target identification (see Mirman, Dixon, & Magnuson, 2008; Mirman, 2014 for a detailed description of this approach). Briefly, GCA uses hierarchically related sub-models to capture the data pattern. The first sub-model, usually called Level 1, captures the effect of time on fixation proportions using fourth-order orthogonal polynomials. Specifically, the intercept term reflects average overall fixation proportion, the linear term reflects a monotonic change in fixation proportion (similar to a linear regression of fixation proportion as a function of time), the quadratic term reflects the symmetric rise and fall rate around a central inflexion point, and the cubic and quartic terms similarly reflect the steepness of the curve around inflexion points and capture additional deflections in the curves. The Level 2 models then, in incremental order, capture the effects of experimental manipulations (as well as differences between participants captured by covariates) on the time terms. The specific order used for each analysis will be described in the Results section. All models were fit using Maximum Likelihood Estimation and compared with a likelihood ratio test using the -2LL deviance statistic (minus 2 times the log-likelihood), which is distributed as χ2 with k degrees of freedom corresponding to the k parameters added. In the current study, step-wise factor-level comparisons were used to evaluate the overall effects of factors in incremental order. This model comparison approach provides the most accurate assessment of statistical significance for fixed effects in such mixed-effect models (Barr, Levy, Scheepers, & Tily, 2013).
4. Results
Both groups were quite accurate in identifying the target images from the visual arrays. Percentage accuracy for trials with and without related distractors in the neutral and action verb contexts is provided in Table 3.
Table 3.
Accuracy for target identification in fillers (without related distractors) and critical trials (with related distractors) for both non-apraxic and apraxic patients.
| Group | Non-apraxic patients | Apraxic patients | ||||||
|---|---|---|---|---|---|---|---|---|
| Trial type | Filler trials | Critical trials | Filler trials | Critical trials | ||||
| Context | SAW | USED | SAW | USED | SAW | USED | SAW | USED |
| Percent accurate (%) | 95.64 | 96.67 | 93.18 | 96.36 | 81.45 | 81.82 | 83.18 | 75.00 |
| Standard deviation | 20.45 | 17.98 | 25.26 | 18.76 | 38.90 | 38.63 | 37.49 | 43.40 |
Fillers: without related distractors; critical trials: with related distractors
Logistic regression analyses were conducted with Accuracy (correct vs. incorrect) as the dependent variable, Group (apraxic vs. non-apraxic), Trial Type (filler vs. critical trials), and Context Verb (Action verb vs. Neutral) along with their two way and three way interactions as predictors, and Total Lesion Volume and WAB comprehension score as covariates. Results showed reliable effects of WAB comprehension score (p<.001) and Group (p<.001), with higher WAB score associated with higher accuracy and apraxic group associated with lower accuracy. These effects, however, did not interact with the effect of Context Verb or the presence of a related distractor (p values ≥0.09).
4.1. Action-related competition effects
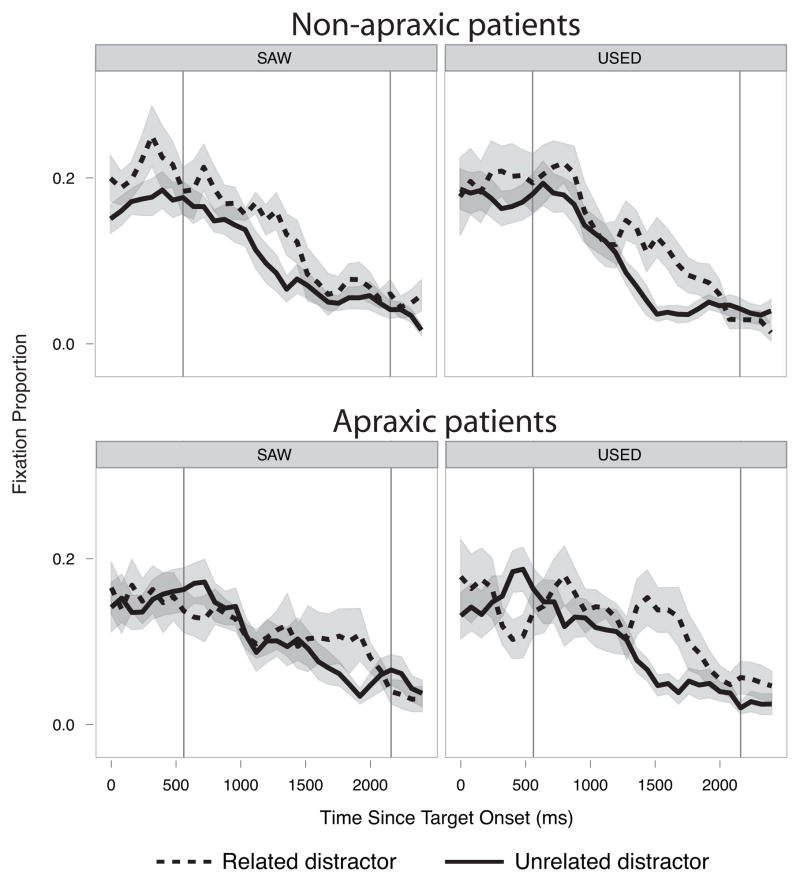
The average fixation proportions to related and unrelated distractors from correct trials were plotted from the onset of the target words and about 2 seconds onward separately for each context for each group (Figure 3).
Figure 3.
Fixation proportion data for related distractors (dashed line) and unrelated distracters (solid line) are plotted for non-apraxic participants (upper panels) and apraxic participants (lower panels) in the neutral “SAW” context on the left and the action verb “USED” context on the right. Ribbons around the data lines show standard errors.
To quantify these results, fixation proportions on distracters from correct trials were examined using GCA. Specifically, the Level 2 models included as fixed effects, in incremental order, the covariates: WAB comprehension score, Total Lesion Volume, and experimental conditions: Action Relatedness (Related vs. Unrelated), Action Relatedness by WAB comprehension score interaction, Action Relatedness by Total Lesion Volume interaction, Context Verb (Action verb vs. Neutral), Action Relatedness by Context Verb interaction, Group (apraxic vs. non-apraxic), Group by Action Relatedness interaction, Group by Context Verb interaction, and finally Group by Action Relatedness by Context Verb interaction. The fixed effect of Group captures any overall difference in the fixation time course across the participant groups. Of greater interest were the interactions with Group, which would capture differential effects of action relatedness between apraxic and non-apraxic participants (Group*Action Relatedness interaction) and whether those differences were modulated by context (Group*Action Relatedness*Context Verb interaction). Details of the model and model fitting results are provided in Appendix A.
The time window for subsequent statistical analyses was defined such that it clearly included effects from both groups. Thus, the time window started at 560ms after target name onset, which was the earliest point at which at least one group’s overall target fixation proportions (averaged across contexts) exceeded chance level (20%), and ended at 2160ms post target onset, at which point the distractor fixation proportions (averaged across contexts) for both groups were below 5%. Note that there are some fixation differences immediately before the target onset, in particular in the “USED” context in the apraxic groups. To test if these fixation differences are reliable, we ran the same analysis on the data −160ms before the target onset and 560ms after the onset (the ‘control’ pre-assessment time window). The results did not yield any significant Group*Action Relatedness*Context Verb interaction (p=0.12), which should eliminate the concern that these fixation differences may be biased by what participants were fixating before the target word.
The results of model fit comparisons showed reliable effects of Action Relatedness (χ2(5) =35.58, p<.0001), as well as a significant three-way interaction between Group, Action Relatedness, and Context Verb (χ2(5) =14.57, p=.01). No other experimental effects were significant. Follow-up between-group comparisons within the “SAW” context showed a significant Action Relatedness effect (χ2(5) =18.69, p <.01), which was modulated by an interaction with the effect of Group (χ2(5) =12.31, p <.05). In the “USED” context, there was also a reliable Action Relatedness effect (χ2(5) =25.55, p =.0001), which, however, did not interact with Group (p=0.2).
To summarize, in the neutral context, there was a significant group difference in the time course of competition effects (fixation of action-related distractors compared to unrelated distractors). These results do not specify the characteristics of this difference, but visual inspection of the fixation curves suggested that competition was delayed in the apraxic relative to the non-apraxic group (Figure 3). However, critically, both groups showed significant action-relatedness competition effects, providing evidence against the hypothesis that action representations are entirely degraded in the apraxic group. Interestingly, the group difference in the competition effect in the neutral context was eliminated in the “USED” context, suggesting that apraxic participants’ access to use-action information can be facilitated by lexical semantic information, as was previously observed in healthy participants (Lee et al, 2012).
To further confirm the link between the observed eye movement effects and apraxics’ deficit in producing use actions and to relate the fixation patterns to their neuroanatomical underpinnings, the following analyses examined the effect of apraxia severity and the neural correlates of the action-related competition effect.
4.2. Differences in action-related competition effect as a function of gesture-to-sight scores
To confirm that the effects observed above were indeed driven by apraxic participants’ deficit in object-associated skilled-used actions, we tested whether the observed effects in the “SAW” context could be accounted for by the severity of apraxia. To that end, participants’ gesture-to-sight scores were added as a continuous variable to a GCA model of the fixation data in the “SAW” context. The contribution of the gesture-to-sight score was assessed by starting with a base model that included WAB comprehension scores, Total Lesion Volume, Action Relatedness (Related vs. Unrelated), Action Relatedness * WAB comprehension score, Action Relatedness * Total Lesion Volume, and Gesture-to-Sight score, and adding the interaction term between the Gesture-to-Sight Score and Action Relatedness. The comparison between the models with and without and interaction term between the Gesture-to-Sight Score and Action Relatedness captures the degree to which individual participants’ Gesture-to-Sight scores modulated the fixation pattern differences between related and unrelated distracters. Details of the model and model fitting results can be found in Appendix B.
The results showed that there was a significant difference between the models with and without the interaction between Gesture-to-Sight Score and Action Relatedness (χ2(5) =20.94, p<.001), indicating a significant improvement of the model fit due to this interaction term, which reflects that Gesture-to-Sight Score modulated the effect of Action Relatedness on distractor fixation proportions. This result corroborates the group effect reported in the previous section and, importantly, extends it by showing that the effect has a graded relation to apraxia severity. This effect amplifies a similar non-significant trend reported by Myung and colleagues (2010).
Having established the association between apraxia severity and delayed action-related competition effect in the neutral verb context, we now turn to the neuroanatomic correlates of the observed abnormalities in apraxic participants’ fixation patterns.
4.3. Neural correlates of differences in action-related competition effect
Exploratory VLSM analysis
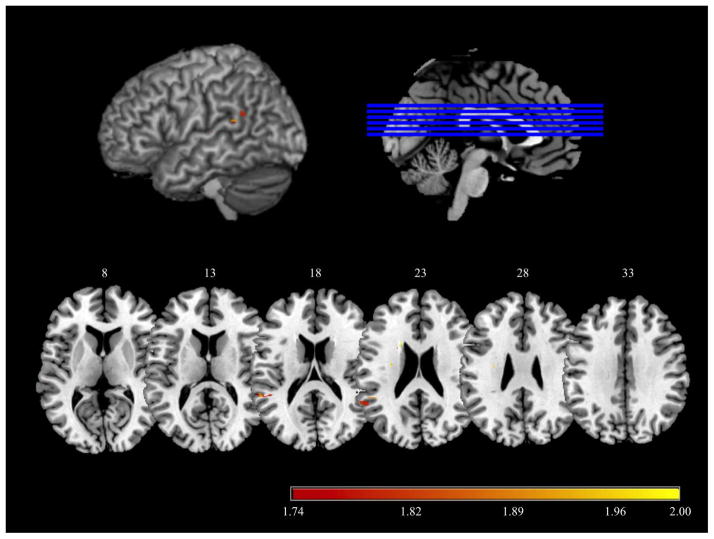
As a first pass to identify voxels that are potentially relevant to the delayed competition effect, we used the VoxBo brain imaging package (www.voxbo.org) to conduct a whole-brain Voxel-Based Lesion Symptom Mapping (VLSM) analysis that used one-tailed t-tests to compare the peak latencies of action-related competition effects in participants with or without lesions at each voxel. The behavioral measure was peak latency of the competition effect in the neutral “SAW” context: the time point (latency) of the maximum difference between related and unrelated distractor fixation proportion, derived from the raw fixation data for each participant. Voxels in which fewer than five participants had a lesion were excluded, leaving 172,490 qualifying voxels in the analysis. Based on previous findings (e.g., Myung et al., 2010) and the current data, only voxels associated with slower competition effects were considered. A lenient criterion was used to define the cortical region of interest, including voxels associated with p values less than 0.05 uncorrected, with a minimum cluster size of 10 voxels (Knutson et al., 2012; Knutson et al., 2013; Lieberman & Cunningham, 2009). In total, this procedure identified 310 voxels, including 120 voxels in BA42, 114 voxels in BA22, 38 voxels in BA41, 33 voxels in BA40, and 5 voxels in BA3, distributed in 4 nearly-contiguous clusters in the posterior superior temporal and inferior parietal lobes (shown in Table 4 and Figure 4). That these clusters are non-contiguous may reflect that there is a degree of between-subject variability in the precise coordinates of the regions subserving incidental activation of action information from viewed objects. (shown in Table 4 and Figure 4). In view of the proximity of these clusters, we combined these VLSM-derived clusters into a single 310-voxel region of interest (ROI).
Table 4.
Information for the 4 clusters derived from the exploratory VLSM analysis, incuding MNI coordinates and t values of the peak voxels, total numbers of volxels and number of voxels in Brodmann’s areas.
| Peak voxel | T | Number of voxels | BA40 | BA3 | BA22 | BA41 | BA42 | ||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||||
| −28 | −43 | 47 | 2.73 | 35 | 30 | 5 | 0 | 0 | 0 |
| −53 | −45 | 22 | 1.89 | 34 | 0 | 0 | 0 | 20 | 14 |
| −58 | −44 | 17 | 1.89 | 116 | 0 | 0 | 0 | 18 | 98 |
| −58 | −53 | 23 | 1.79 | 125 | 3 | 0 | 114 | 0 | 8 |
Figure 4.
Exploratory VLSM statistical t map overlaid on a MNI brain. Regions associated with differences in peak latency with related versus unrelated distractors (the “competition effect”) in the neutral context are shown in warm colors, thresholded at p < 0.05, uncorrected (t > 1.74).
GCA with percent damage
To validate the relation between impairments in this ROI identified from raw peak fixation data and the continuous time course of the eye movement competition effect, we conducted the following analysis. Using the same analysis strategy employed with Gesture-to-Sight scores (above), we examined whether proportional damage in the neuroanatomic ROI improved the fit of a GCA model of fixation time course. Specifically, the contribution of percent damage was evaluated by the improvement in model fit due to adding the interaction term between the percentage damage and Action Relatedness to a base model that included the covariates WAB comprehension scores, Total Lesion Volume, the factors Action Relatedness (Related vs. Unrelated), Action Relatedness * WAB comprehension score, Action Relatedness * Total Lesion Volume, and percent damage itself. The results showed that percent damage in this ROI indeed significantly improved model fit for fixation differences due to action relatedness (χ2(5) =17.66, p<.01), indicating that the amount of damage in a relatively small posterior superior temporal and inferior parietal region (over and above total lesion volume and language comprehension severity) modulated the time course of differences in fixations to action-related as compared to unrelated distractors. See Appendix C for the details of the model and model fitting results.
5. Discussion
In this study we demonstrated a three-way interaction between effects of Group, Action Relatedness, and Context Verb when participants with or without apraxia locate a target object in an array with use-related and unrelated distractors. Specifically, our results showed that apraxic participants, like non-apraxic stroke patients and healthy participants (Lee et al., 2012), fixate more on distractors that are related to the target in skilled-use action features than on unrelated distractors, suggesting that apraxic participants’ skilled-use action knowledge is not entirely lost. Nonetheless, apraxics differed from non-apraxics in showing a later emerging competition effect in a neutral context. This suggests that apraxia is associated with abnormalities in the speed with which action knowledge is activated during object processing. Moreover, extending the results of Myung et al. (2010), we demonstrated that these group differences remain even after accounting for patients’ overall severity, as indexed by lesion volume and comprehension deficits. Furthermore, the magnitude of the competition abnormalities were reliably predicted by the severity of participants’ deficits on a clinical apraxia test, gesture-to-sight of objects. Finally, we demonstrated that incidental activation of skilled-use action information in apraxic participants was facilitated when the context contained an action verb, “used”. On a number of prominent accounts, cueing effects have been attributed to deficits in semantic access rather than in the integrity of semantic representations (for a review, see Mirman & Britt, 2014; for similar arguments, see Auchterlonie et al., 2002; Brambati et al., 2012; Corbett et al., 2011; Jefferies et al., 2007; Jefferies et al., 2008; Jefferies & Lambon Ralph, 2006; Tyler & Ostrin, 1994; Warrington & Shallice, 1979). On such accounts, the delayed availability of use action information in the neutral “SAW” context and the normalization of performance in the “USED” context are consistent with a deficit in access to object use representations.
Our finding that the degree of apraxia on an explicit clinical test is predictive of degree of abnormality in incidental activation of object-related action has implications for ‘grounded’ theories of cognition. These results indicate that spatiomotor deficits and lesions to brain regions encoding complex object-related spatiomotor actions can affect object concepts. In addition, the results suggest that under the real-time constraints of naturalistic action, apraxics’ slowed activation of action knowledge may contribute to apraxic errors. Moreover, the finding that apraxics’ access to skilled-use action information can be primed suggests that future emphasis should be placed on understanding the role of context in the activation of action-related information from objects. We will now expand on each of these points in turn.
5.1. Apraxia and temporal-parietal lesions modulate the activation of object-associated action information
The apraxia literature contains numerous accounts of the underlying deficit in apraxia. Classic accounts describe ideational apraxia as an “agnosia of usage”; that is, a circumscribed deficit in knowledge of action characterized by substitutions, omissions, and reversals in naturalistic tasks such as coffee-making (Morlaas, 1928; Renzi & Lucchelli, 1988). Ideomotor apraxia, in contrast, has been characterized by spatio-temporal and postural errors in single object-use and imitation tasks, reflecting putative damage to stored “space-time-form” representations of object use movements (Geschwind, 1975; Heilman & Rothi, 1993; Liepmann, 1905). In practice, the characteristics traditionally used to define these two putative subtypes frequently co-occur. Reflecting this, apraxia in the present study was characterized based on postural and dynamic (amplitude/timing) errors on a test of gesture to the sight of objects, frequently associated with ideomotor apraxia. Our findings demonstrated that performance on this apraxia test is reliably correlated with a measure of the incidental activation of action information from viewed objects. Thus, consistent with previous suggestions (e.g., Buxbaum, Kyle, & Menon, 2005), “knowledge” of object-related actions is associated with the ability to perform these actions. This is a substantial refinement of the classic account of apraxia as an “agnosia of usage” (Morlaas, 1928).
In this study, patients’ lesion information was obtained with different imaging methods due to contraindications to the MR environment for some patients. Although the distributions of imaging types did not differ by apraxia designation (see page 16 for more detailed information) and therefore should not bias our results, these method differences may have introduced noise in both the segmentation and analysis of the lesions, and may have weakened our chances of observing statistically significant results.
Nevertheless, our exploratory lesion analyses suggested a relationship between the amount of damage to several circumscribed clusters of voxels in the left parietal and temporal cortices, on the one hand, and latency of manipulation activation, on the other, even after controlling for stroke severity and overall lesion volume. These clusters are localized within brain regions with a recognized role in action processing. Together, posterior temporal and inferior parietal cortex, including left posterior middle and superior temporal gyrus and supramarginal gyrus, form one of the major regions consistently activated in studies requiring access to conceptual representations of action (see Watson, Cardillo, Ianni, & Chatterjee, 2013 for a meta-analysis). Superior temporal cortical regions have been shown to be active during processing of biological motions (Giese & Poggio, 2003; Puce & Perrett, 2003), object-directed actions (James, VanDerKlok, Stevenson, & James, 2011), and moving objects in interactions that appear to be causal or intentional (Blakemore et al., 2003; Castelli, Happé, Frith, & Frith, 2000; Schultz, Friston, O’Doherty, Wolpert, & Frith, 2005). The left posterior superior temporal cortex has also been shown to be active during semantic judgments about action words relative to object words and is considered critical in representing abstract aspects of action concepts that are accessible by language (Chatterjee, 2008; Kable, Kan, Wilson, Thompson-Schill, & Chatterjee, 2005). In addition, lateral temporal cortex has also been shown to be critical in understanding thematic roles in action events (Wu, Waller, & Chatterjee, 2007). The left IPL is also an important locus of object-related action representations (e.g., Boronat et al., 2005; Buxbaum, Kyle, Tang, & Detre, 2006; Ishibashi, Lambon Ralph, Saito, & Pobric, 2011; Goldenberg & Spatt, 2009; Kellenbach et al., 2003; Vingerhoets, Acke, Vandemaele, & Achten, 2009). A number of studies have demonstrated that the supramarginal gyrus (SMG, BA40) mediates hand postures appropriate for functional tool use (e.g., Bach, Peelen, & Tipper, 2010; Pelgrims, Olivier, & Andres, 2011). Peeters, Orban & Rizzolatti (2013) recently demonstrated that the SMG is activated not only by tool-use actions, but also by hand actions performed with the same kinematics as a tool (e.g., movement of the hand in a “raking” action), suggesting that this region processes dynamic information to plan moving a tool to obtain an intended result. The results of the present study suggest that temporal-parietal regions are recruited, as well, in incidental activation of action information when objects are processed semantically, even when such processing is task-irrelevant. However, our sample of participants was admittedly small for a robust analysis of neuroanatomic correlates of the observed behavioral effects, and future studies should attempt to replicate and extend these findings with a larger sample size.
The observed delays in incidental activation of action during object processing in temporal-parietal apraxia has important implications for ‘grounded’ theories of cognition. Grounded cognition theories suggest that retrieving a concept involves activation of the sensory or motor attributes associated with the concept (Bischoff et al., 2012). These theories have been supported by findings that show activation of action-related brain regions during processing of manipulable artifacts (Chao & Martin, 2000; Martin, Wiggs, Ungerleider, & Haxby, 1996) or verbs (e.g., Pulvermüller, 2005; Sakreida, Scorolli, Menz, Heim, & Borghi, 2013; Tettamanti et al., 2011). Given that activations in functional neuroimaging studies may be epiphenomenal to conceptual processing, important converging evidence for ‘groundedness’ comes from recent demonstrations that performance of an irrelevant action task may interfere specifically with tool identification (Witt, Kemmerer, Linkenauger, & Culham, 2010; Yee, Chrysikou, Hoffman, & Thompson-Schill, 2013), and that tactile or proprioceptive stimulation to the hands facilitates conceptual processing of small, manipulable objects (but not larger objects) relative to control stimulation. The present findings of a graded relationship between apraxia severity and incidental action information during object processing add to the body of evidence suggesting that manipulable objects are represented in part in terms of the actions that are associated with them.
It is likely that a critical component of incidental activation of action information from objects may be the ability to generate motor imagery (i.e., action simulation) (e.g., Frak, Nazir, Goyette, Cohen, & Jeannerod, 2010; Jeannerod, 2001). Action simulation has been widely proposed to underlie action recognition (e.g., Grafton, 2009; Griffiths & Tipper, 2012; Tidoni, Borgomaneri, Pellegrino, & Avenanti, 2013) and processing of action verbs (e.g., Borghi & Scorolli, 2009; Springer, Huttenlocher, & Prinz, 2012). Simulation has also been proposed to play a role in object processing (Tipper, Paul, & Hayes, 2006). Apraxics are deficient in generating internal representations of action, and rely abnormally upon visual feedback (Buxbaum et al., 2005; Haaland et al., 2000; Jax et al., 2006). Moreover, lesions in the IPL are associated with a deficit in this motor simulation capacity (Buxbaum et al., 2005; Sirigu et al., 1996). In keeping with these findings, both the behavioral and neuroanatomic data from the present study are consistent with the proposal that manipulable object representations are grounded in sensorimotor capacity, and that spatiomotor deficits (and temporal-parietal lesions) may causally affect object representations.
The association of apraxic errors on a clinical task, damage to brain regions known to mediate object use, and delayed incidental activation of specific hand posture information when objects are viewed speak against a recent account suggesting that tool manipulation knowledge is not stored, but rather recreated de novo each time a tool is encountered based on “technical reasoning”. On this account, apraxia results from “difficulties in identifying (distinction) and unifying (sequenciation) the technical means (dense, permeable, resistant, etc.) relevant for a given technical end (cutting, engraving, etc.)” (Jarry, Osiurak, et al. 2013). On such an account, the present results, along with data showing that some apraxics can rapidly activate grasp hand postures required to move objects, but not use hand postures (Jax & Buxbaum, 2013), would require that only use hand postures (and not grasp hand postures) are slowed as a result of slowed technical means-end reasoning. Moreover, this account would require that defective technical means-end reasoning is incidentally (and rapidly) attempted on-line even in tasks (such as word-picture matching) in which it is entirely irrelevant. The more parsimonious account is simply that task-irrelevant simulations of skilled object use are slowed. However, it is also important to note that the two accounts are not mutually exclusive; that is, the complex syndrome of apraxia may indeed reflect varying degrees of deficit in technical reasoning, along with deficits in rapid activation of stored object-use information. Just as there is not a single subtype of aphasia, it is possible that there are distinct apraxia subtypes in which stored versus “on-line” processes make differing contributions. Future studies would benefit from moving beyond the claim that apraxia can be reduced to a single deficit, instead considering the contribution of carefully-defined deficits as a function of lesion location and pattern of apraxia performance.
5.2. An action verb context may facilitate apraxics’ retrieval of action information
Our results indicate that although apraxic participants showed delayed action competition effects in the neutral verb “SAW” context, their eye-fixation competition effects were statistically equivalent to those of non-apraxic control participants with provision of the action verb “USED”. This suggests a facilitative role of action verb context in apraxics’ action retrieval. As noted earlier, neurologically intact participants performing the present experimental task also showed reliable facilitation of action information in the “USED” context (Lee et al., 2012). Additionally, we previously demonstrated in similar eyetracking studies with neurologically intact participants that provision of a sentential context (e.g., “he wanted to prepare breakfast”) drives eyegaze to goal-related related distractor objects (e.g., coffeemaker) (Kalénine, Mirman, Middleton, & Buxbaum, 2012). These findings echo those of other investigators (e.g., Schuil, Smits, & Zwaan, 2013) in suggesting that activation of action information may be modulated by context.
The present data raise the question of the degree to which modulation of action information by verbs may be of therapeutic benefit to individuals with apraxia. In both clinical and research contexts, apraxics are not infrequently asked to “show how to use that [object]”, to little benefit. Previous investigations indicate that verb processing is associated with motor and premotor activation (e.g., Buccino et al., 2005; Hauk, Johnsrude, & Pulvermüller, 2004). We have suggested previously (Lee et al., 2012) that the enhanced competition effects we observed in the “USED” context may occur through the mediation of these regions. It is possible that the motor resonance from the verb phrase observed in the present study (e.g., “used the stapler”) is sufficient to influence attention and eye movements, but not sufficient to drive the motor system with the strength or specificity necessary to facilitate manual action programming (c.f. Jeannerod, 2001 for discussion of the relation between simulation and overt actions). In view of this, future emphasis in apraxia rehabilitation should be placed on facilitating the motor activation that occurs when objects are viewed, perhaps by strengthening linkages between object-related action and other contextual information, such as the verbs, people, places, and sounds associated with these actions (see Smania, Aglioti, et al., 2006).
6. Conclusion
The present study has demonstrated that patients with deficient performance on tests of pantomime to the sight of objects show abnormalities in the robustness with which action information is incidentally activated when manipulable objects are viewed. Nevertheless, activation of action information was responsive to a cueing manipulation, suggesting that the impairment is similar to those that have been described as an “access” deficit. Moreover, we showed that these deficits cannot be attributed to overall severity or to language comprehension impairments. On the other hand, we provided evidence that the deficits are associated with proportion damage to a region in the posterior superior temporal and inferior parietal lobes. Because deficits in pantomime and on tests of gesture recognition are correlated, the participants are likely to be impaired in both conceptual and production-related aspects of object-related action, as is the case with many patients with left hemisphere stroke. In future investigations it will be of interest to assess whether deficient incidental access to action information is evident in patients for whom traditional explicit measures of gesture knowledge, such as gesture recognition, are completely normal, or whether the incidental-access deficit is reserved for apraxic patients in whom traditionally-assessed gesture knowledge is impaired.
Highlights.
Apraxics show slowed incidental access to use-action knowledge during object processing.
Lower apraxia scores predict slower access to use-action knowledge.
Larger lesions in IPL and pSTG predict slower access to use-action knowledge.
Context containing an action verb facilitates apraxics' access to action knowledge.
These data substantially refine accounts of action semantics deficits in apraxia.
Acknowledgments
The authors wish to thank Solène Kalénine, Steven Jax, and Branch Coslett for their helpful suggestions during manuscript preparation, Allison Shapiro and Branch Coslett for their help with lesion segmentation and registration, and Allison Shapiro and Rachel German for assistance with data collection. This study was supported by a NIH grant R01 NS065049 and James S. McDonnell Foundation #220020190 to Laurel J. Buxbaum, a NIH grant R01 DC010805 to Daniel Mirman, and a Taiwan National Science Council grant NSC102-2410-H-002-055 to Chia-lin Lee.
Appendix Growth curve analysis
Model structures
The base model shows the complete set of time terms and random effects. The subsequent comparison models show the incrementally added fixed effects where “Time” stands for the complete set of time terms up to the quartic term and “*” is compact notation indicating the complete set of main effects and interactions. The base model random effects were kept constant for all subsequent comparison models. For each model, the incrementally added terms are underlined.
Note: T1: linear time term; T2: Quadratic time term; T3: Cubic time term; T4: Quartic time term; WAB: WAB comprehension score; LV: Total Lesion Volume; Sub: subject; VC: Verb Context; AR: Action Relation; Group: Patient Group; G2S: Gesture-to-Sight score; PD: Percent Damage
Model fitting results
Parameter estimates for the full model and results of the χ2 tests for the improvement in model fit due to adding each term to the model.
A. Action competition effect
Model structures
Base model: Fixation ~ (T1+T2+T3+T4)+( T1+T2|Sub*VC*AR)+( T1+T2+T3+T4|Sub)
Model2.1: (Time)* (WAB)
Model2.2: (Time)*(LV+WAB)
Model2.3: (Time)*(AR+LV+WAB)
Model2.4: (Time)*(AR*LV+WAB)
Model2.5: (Time)*(AR*WAB+AR*LV)
Model2.6: (Time)*(VC+AR*WAB+AR*LV)
Model2.7: (Time)*(AR*VC+AR*WAB+AR*LV)
Model2.8: (Time)*(Group+AR*VC+AR*WAB+AR*LV)
Model2.9: (Time)*(Group*AR+AR*VC+AR*WAB+AR*LV)
Model2.10: (Time)*(Group*VC+Group*AR+AR*VC+AR*WAB+AR*LV)
Model2.11: (Time)*(Group*AR*VC+AR*WAB+AR*LV)
Model fitting results
| Intercept | Linear | Quadratic | Cubic | Quartic | χ2 | df | p | |
|---|---|---|---|---|---|---|---|---|
| WAB | 0.0000 (0.00) | −0.0916 (0.11) | 0.0298 (0.1) | 0.1187 (0.04) | 0.0667 (0.04) | 13.38 | 5 | 0.0201 |
| LV | 0.0000 (0.00) | −0.0837 (0.03) | −0.0021 (0.03) | −0.0155 (0.03) | −0.0023 (0.01) | 0.00 | 5 | 1.0000 |
| AR | −0.2912 (0.09) | 0.0292 (0.02) | −0.0098 (0.01) | 0.0000 (0.00) | 0.0422 (0.02) | 35.58 | 5 | 0.0000 |
| AR*WAB | 0.027 (0.07) | −0.0547 (0.07) | −0.04 (0.07) | 0.0395 (0.02) | 0.0000 (0.00) | 27.13 | 5 | 0.0001 |
| AR*LV | 0.0613 (0.08) | 0.0354 (0.08) | 0.0276 (0.07) | −0.0523 (0.02) | 0.0000 (0.00) | 1.56 | 5 | 0.9055 |
| VC | −0.3467 (0.2) | 0.0381 (0.18) | 0.1069 (0.08) | −0.0582 (0.07) | −0.0211 (0.02) | 4.21 | 5 | 0.5198 |
| AR*VC | 0.0272 (0.09) | −0.0131 (0.02) | 0.0042 (0.01) | 0.0000 (0.00) | 0.0209 (0.02) | 6.44 | 5 | 0.2656 |
| Group | 0.0019 (0.05) | −0.0011 (0.01) | −0.0211 (0.01) | 0.0000 (0.00) | −0.0141 (0.02) | 8.58 | 5 | 0.1269 |
| Group*AR | 0.458 (0.25) | −0.047 (0.05) | 0.0162 (0.02) | 0.0000 (0.00) | −0.0099 (0.06) | 8.29 | 5 | 0.1411 |
| Group*VC | −0.011 (0.02) | 0.0033 (0.02) | 0.0029 (0.00) | 0.0000 (0.00) | 0.0545 (0.06) | 8.07 | 5 | 0.1523 |
| Group*AR*VC | −0.4263 (0.23) | 0.0287 (0.05) | 0.0021 (0.02) | 0.0000 (0.00) | −0.0226 (0.02) | 14.57 | 5 | 0.0124 |
B. Gesture-to-Sight scores and action competition effects in the “SAW” context Model structures
Base model: Fixation ~ (T1+T2+T3+T4)*(AR*WAB+AR*LV) +( T1+T2|Sub*VC*AR)+( T1+T2+T3+T4|Sub)
Model2.1: (Time)* (G2S+AR*WAB+ AR*LV)
Model2.2: (Time)* (G2S*AR+G2S+AR*WAB+ AR*LV)
Model fitting results
| Intercept | Linear | Quadratic | Cubic | Quartic | χ2 | df | p | |
|---|---|---|---|---|---|---|---|---|
| G2S | 0.0005 (0.00) | −0.0006 (0.00) | 0.0008 (0.00) | −0.0007 (0.00) | −0.0004 (0.00) | 5.13 | 5 | 0.4005 |
| G2S*AR | 0.0000 (0.00) | −0.0017 (0.00) | −0.0021 (0.00) | 0.0029 (0.00) | 0.0021 (0.00) | 20.94 | 5 | 0.0008 |
C. Percentage damage and action competition effects in the “SAW” context Model structures
Base model: Fixation ~ (T1+T2+T3+T4)*(AR*WAB+AR*LV) +( T1+T2|Sub*VC*AR)+( T1+T2+T3+T4|Sub)
Model2.1: (Time)* (PD+AR*WAB+ AR*LV)
Model2.2: (Time)* (PD*AR+PD+AR*WAB+ AR*LV)
Model fitting results
| Intercept | Linear | Quadratic | Cubic | Quartic | χ2 | df | p | |
|---|---|---|---|---|---|---|---|---|
| PD | 0.0384 (0.03) | −0.0217 (0.1) | 0.0101 (0.07) | −0.0288 (0.05) | 0.0474 (0.04) | 0.00 | 25 | 1.0000 |
| PD*AR | −0.0227 (0.02) | 0.2156 (0.11) | −0.1887 (0.09) | −0.0477 (0.04) | −0.1214 (0.04) | 5.18 | 5 | 0.3945 |
Footnotes
As noted earlier, some theoretical perspectives attempt to distinguish ideational and ideomotor apraxia subtypes. However, there is substantial variability across research groups in the characteristics said to define each type (see Buxbaum, 2001, for review). Consequently, we use the generic term “apraxia” to describe the patients in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allport D. Current Perspectives in Dysphasia. Edinburgh: Churchill Livingstone; 1985. Distributed memory, modular subsystems and dysphasia; pp. 207–244. [Google Scholar]
- Auchterlonie S, Phillips NA, Chertkow H. Behavioral and electrical brain measures of semantic priming in patients with Alzheimer’s disease: Implications for access failure versus deterioration hypotheses. Brain and Cognition. 2002;48(2–3):264–267. [PubMed] [Google Scholar]
- Avants BB, Schoenemann PT, Gee JC. Lagrangian frame diffeomorphic image registration: Morphometric comparison of human and chimpanzee cortex. Medical Image Analysis. 2006;10(3):397–412. doi: 10.1016/j.media.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bach P, Peelen MV, Tipper SP. On the role of object information in action observation: an fMRI study. Cerebral Cortex (New York, NY: 1991) 2010;20(12):2798–2809. doi: 10.1093/cercor/bhq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri C, De Renzi E. The executive and ideational components of apraxia. Cortex. 1988;24(4):535–543. doi: 10.1016/s0010-9452(88)80047-9. [DOI] [PubMed] [Google Scholar]
- Barr DJ, Levy R, Scheepers C, Tily HJ. Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language. 2013;68(3):255–278. doi: 10.1016/j.jml.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou LW. Grounded Cognition. Annual Review of Psychology. 2008;59(1):617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends in Cognitive Sciences. 2011;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Boyer P, Pachot-Clouard M, Meltzoff A, Segebarth C, Decety J. The detection of contingency and animacy from simple animations in the human brain. Cerebral Cortex (New York, NY : 1991) 2003;13(8):837–844. doi: 10.1093/cercor/13.8.837. [DOI] [PubMed] [Google Scholar]
- Blumstein SE, Milberg W, Shrier R. Semantic processing in aphasia: Evidence from an auditory lexical decision task. Brain and Language. 1982;17(2):301–315. doi: 10.1016/0093-934x(82)90023-2. [DOI] [PubMed] [Google Scholar]
- Borghi AM, Scorolli C. Language comprehension and dominant hand motion simulation. Human Movement Science. 2009;28(1):12–27. doi: 10.1016/j.humov.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Boronat CB, Buxbaum LJ, Coslett HB, Tang K, Saffran EM, Kimberg DY, Detre JA. Distinctions between manipulation and function knowledge of objects: evidence from functional magnetic resonance imaging. Brain Research Cognitive Brain Research. 2005;23(2–3):361–373. doi: 10.1016/j.cogbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Bouillaud J-B. Migneret. 1825. Recherches cliniques propres à démontrer que la perte de la parole correspond à la lésion des lobules antérieurs du cerveau, et à confirmer l’opinion de Gall, sur le siège du langage articulé. [Google Scholar]
- Brambati SM, Peters F, Belleville S, Joubert S. Lack of semantic priming effects in famous person recognition in Mild Cognitive Impairment. Cortex. 2012;48(4):414–420. doi: 10.1016/j.cortex.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Buccino, Riggio L, Melli G, Binkofski F, Gallese V, Rizzolatti G. Listening to action-related sentences modulates the activity of the motor system: a combined TMS and behavioral study. Brain Research Cognitive Brain Research. 2005;24(3):355–363. doi: 10.1016/j.cogbrainres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Johnson-Frey SH, Bartlett-Williams M. Deficient internal models for planning hand–object interactions in apraxia. Neuropsychologia. 2005;43(6):917–929. doi: 10.1016/j.neuropsychologia.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Veramonti T, Schwartz MF. Function and manipulation tool knowledge in apraxia: Knowing “what for” but not “how. Neurocase. 2000;6(2):83–97. [Google Scholar]
- Buxbaum LJ, Kyle KM, Menon R. On beyond mirror neurons: internal representations subserving imitation and recognition of skilled object-related actions in humans. Brain Research Cognitive Brain Research. 2005;25(1):226–239. doi: 10.1016/j.cogbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ. Ideomotor apraxia: a call to action. Neurocase. 2001;7:445–458. doi: 10.1093/neucas/7.6.445. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Coslett HB. Spatio-motor representations in reaching: Evidence for subtypes of optic ataxia. Cognitive Neuropsychology. 1998;15(3):279–312. doi: 10.1080/026432998381186. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Grossman M, Coslett HB. Left inferior parietal representations for skilled hand-object interactions: Evidence from stroke and corticobasal degeneration. Cortex. 2007;43(3):411–23. doi: 10.1016/s0010-9452(08)70466-0. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Tang K, Detre JM. Neural substrates of knowledge of hand postures for object grasping and functional object use: Evidence from fMRI. Brain Research. 2006;1117(1):175–185. doi: 10.1016/j.brainres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Saffran EM. Knowledge of object manipulation and object function: Dissociations in apraxic and non-apraxic subjects. Brain and Language. 2002;82:179–199. doi: 10.1016/s0093-934x(02)00014-7. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Sirigu A, Schwartz MF, Klatzky R. Cognitive representations of hand posture in ideomotor apraxia. Neuropsychologia. 2003;41:1091–1113. doi: 10.1016/s0028-3932(02)00314-7. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Veramonti T, Schwartz MF. Function and manipulation tool knowledge in apraxia: Knowing “what for” but not “how. Neurocase. 2000;6(2):83–97. [Google Scholar]
- Campanella F, Shallice T. Refractoriness and the healthy brain: A behavioural study on semantic access. Cognition. 2011a;118(3):417–431. doi: 10.1016/j.cognition.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Campanella F, Shallice T. Manipulability and object recognition: is manipulability a semantic feature? Experimental Brain Research. 2011b;208(3):369–383. doi: 10.1007/s00221-010-2489-7. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50(3):1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Happé F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12(3):314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Chao L, Martin A. Representation of manipulable man-made objects in teh dorsal stream. Neuroimage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. The neural organization of spatial thought and language. Seminars in Speech and Language. 2008;29(3):226–252. doi: 10.1055/s-0028-1082886. [DOI] [PubMed] [Google Scholar]
- Clark MA, Merians AS, Kothari A, Poizner H, Macauley B, Rothi LJG, Heilman KM. Spatial planning deficits in limb apraxia. Brain. 1994;117(5):1093–1106. doi: 10.1093/brain/117.5.1093. [DOI] [PubMed] [Google Scholar]
- Corbett F, Jefferies E, Lambon Ralph MA. Deregulated semantic cognition follows prefrontal and temporo-parietal damage: evidence from the impact of task constraint on nonverbal object use. Journal of Cognitive Neuroscience. 2011;23(5):1125–1135. doi: 10.1162/jocn.2010.21539. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Category-related recognition defects as a clue to the neural substrates of knowledge. Trends in the Neurosciences. 1990;13:95–98. doi: 10.1016/0166-2236(90)90184-c. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Lucchelli F. Are semantic systems separately represented in the brain? The case of living category impairment. Cortex. 1994;30(1):3–25. doi: 10.1016/s0010-9452(13)80322-x. [DOI] [PubMed] [Google Scholar]
- Di Pellegrino G, Rafal R, Tipper SP. Implicitly evoked actions modulate visual selection: evidence from parietal extinction. Current Biology. 2005;15(16):1469–1472. doi: 10.1016/j.cub.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Faglioni P, Basso A, Botti C, Aglioti S, Saetti C. Attention and performance. XIII. Motor representation and control. Hillsdale (NJ): Lawrence Erlbaum; 1990. Gestural learning and apraxia; pp. 837–56. [Google Scholar]
- Frak V, Nazir T, Goyette M, Cohen H, Jeannerod M. Grip force is part of the semantic representation of manual action verbs. PLoS ONE. 2010;5(3):e9728. doi: 10.1371/journal.pone.0009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea Frank E, Dombovy Mary, Mahon Bradford Z. Preserved tool knowledge in the context of impaired action knowledge: implications for models of semantic memory. Frontiers in human neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. The Apraxias: neural mechanisms of disorders of learned movement: the anatomical organization of the language areas and motor systems of the human brain clarifies apraxic disorders and throws new light on cerebral dominance. American Scientist. 1975;63(2):188–195. [PubMed] [Google Scholar]
- Giese MA, Poggio T. Neural mechanisms for the recognition of biological movements. Nature Reviews Neuroscience. 2003;4(3):179–192. doi: 10.1038/nrn1057. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Apraxia and the parietal lobes. Neuropsychologia. 2009;47(6):1449–1459. doi: 10.1016/j.neuropsychologia.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Spatt J. The neural basis of tool use. Brain. 2009;132(6):1645–1655. doi: 10.1093/brain/awp080. [DOI] [PubMed] [Google Scholar]
- Grafton ST. Embodied cognition and the simulation of action to understand others. Annals of the New York Academy Sciences. 2009;1156(1):97–117. doi: 10.1111/j.1749-6632.2009.04425.x. [DOI] [PubMed] [Google Scholar]
- Green C, Hummel JE. Familiar interacting object pairs are perceptually grouped. Journal of Experimental Psychology: Human Perception and Performance. 2006;32(5):1107–1119. doi: 10.1037/0096-1523.32.5.1107. [DOI] [PubMed] [Google Scholar]
- Griffiths D, Tipper SP. When far becomes near: shared environments activate action simulation. Quarterly Journal of Experimental Psychology (2006) 2012;65(7):1241–1249. doi: 10.1080/17470218.2012.688978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123(Pt 11):2306–2313. doi: 10.1093/brain/123.11.2306. [DOI] [PubMed] [Google Scholar]
- Haaland Kathleen Y, Flaherty D. The different types of limb apraxia errors made by patients with left vs. right hemisphere damage. Brain and Cognition. 1984;3(4):370–384. doi: 10.1016/0278-2626(84)90029-0. [DOI] [PubMed] [Google Scholar]
- Haaland Kathleen Y, Harrington DL, Knight RT. Spatial deficits in ideomotor limb apraxia A kinematic analysis of aiming movements. Brain. 1999;122(6):1169–1182. doi: 10.1093/brain/122.6.1169. [DOI] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermüller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41(2):301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Rothi LJG. Apraxia. Clinical Neuropsychology. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. New York: Oxford University Press; 1993. pp. 141–150.pp. 141–150. [Google Scholar]
- Heilman KM, Rothi LJG, Valenstein E. Two forms of Ideomotor Apraxia. Neurology. 1982;32(4):342–342. doi: 10.1212/wnl.32.4.342. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of computer assisted tomography. 1998;22(2):324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Huettig F, Altmann GTM. Word meaning and the control of eye fixation: semantic competitor effects and the visual world paradigm. Cognition. 2005;96(1):B23–B32. doi: 10.1016/j.cognition.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Huettig F, Altmann GTM. Visual-shape competition during language-mediated attention is based on lexical input and not modulated by contextual appropriateness. Visual Cognition. 2007;15:985–1018. [Google Scholar]
- Ishibashi R, Lambon Ralph MA, Saito S, Pobric G. Different roles of lateral anterior temporal lobe and inferior parietal lobule in coding function and manipulation tool knowledge: evidence from an rTMS study. Neuropsychologia. 2011;49(5):1128–1135. doi: 10.1016/j.neuropsychologia.2011.01.004. [DOI] [PubMed] [Google Scholar]
- James TW, VanDerKlok RM, Stevenson RA, James KH. Multisensory perception of action in posterior temporal and parietal cortices. Neuropsychologia. 2011;49(1):108–114. doi: 10.1016/j.neuropsychologia.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarry C, Osiurak F, Delafuys D, Chauviré V, Etcharry-Bouyx F, Le Gall D. Apraxia of tool use: More evidence for the technical reasoning hypothesis. Cortex. 2013;49(9):2322–33. doi: 10.1016/j.cortex.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Jax SA, Buxbaum LJ. Response interference between functional and structural object related actions is increased in patients with ideomotor apraxia. Journal of neuropsychology. 2013;7(1):12–18. doi: 10.1111/j.1748-6653.2012.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jax SA, Buxbaum LJ, Moll AD. Deficits in movement planning and intrinsic coordinate control in Ideomotor Apraxia. Journal of Cognitive Neuroscience. 2006;18(12):2063–2076. doi: 10.1162/jocn.2006.18.12.2063. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage. 2001;14(1):S103–S109. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Baker SS, Doran M, Ralph MAL. Refractory effects in stroke aphasia: a consequence of poor semantic control. Neuropsychologia. 2007;45(5):1065–1079. doi: 10.1016/j.neuropsychologia.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph M. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129(8):2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Lambon Ralph MA. Deficits of knowledge vs. executive control in semantic cognition: Insights from cued naming. Neuropsychologia. 2008;46(2):649–658. doi: 10.1016/j.neuropsychologia.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Kan IP, Wilson A, Thompson-Schill SL, Chatterjee A. Conceptual representations of action in the lateral Temporal cortex. Journal of Cognitive Neuroscience. 2005;17(12):1855–1870. doi: 10.1162/089892905775008625. [DOI] [PubMed] [Google Scholar]
- Kalénine S, Buxbaum LJ, Coslett HB. Critical brain regions for action recognition: lesion symptom mapping in left hemisphere stroke. Brain. 2010;133(11):3269–3280. doi: 10.1093/brain/awq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalénine S, Mirman D, Buxbaum LJ. A combination of thematic and similarity-based semantic processes confers resistance to deficit following left hemisphere stroke. Frontiers in Human Neuroscience. 2012;6:106. doi: 10.3389/fnhum.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalénine S, Mirman D, Middleton EL, Buxbaum LJ. Temporal dynamics of activation of thematic and functional knowledge during conceptual processing of manipulable artifacts. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2012;38(5):1274–95. doi: 10.1037/a0027626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenbach ML, Brett M, Patterson K. Actions speak louder than functions: The importance of manipulability and action in tool representation. Journal of Cognitive Neuroscience. 2003;15(1):30–46. doi: 10.1162/089892903321107800. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia battery test manual. [manual] The Psychological Corporation; 1982. [Google Scholar]
- Knutson KM, Krueger F, Dal Monte O, Raymont V, Snyder AD, Kirsch HE, Wassermann EM, Grafman J. Gustatory cortical lesions affect motivation for snack foods. Cognitive Neuroscience. 2012;3(2):131–138. doi: 10.1080/17588928.2012.688018. [DOI] [PubMed] [Google Scholar]
- Knutson Kristine M, Rakowsky ST, Solomon J, Krueger F, Raymont V, Tierney MC, Wassermann EM, Grafman J. Injured brain regions associated with anxiety in Vietnam veterans. Neuropsychologia. 2013;51(4):686–694. doi: 10.1016/j.neuropsychologia.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CL, Middleton E, Mirman D, Kalénine S, Buxbaum LJ. Incidental and context-responsive activation of structure- and function-based action features during object identification. Journal of Experimental Psychology: Human Perception and Performance. 2012;39 (1):257–270. doi: 10.1037/a0027533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepmann H. The left hemisphere and action. London, Ontario: University of Western Ontario; 1905. [Google Scholar]
- Lordat J. Analyse de la parole, pour servir à la théorie de divers cas d’alalie et de paralalie (de mutisme et d’imperfection du parler) que les nosologistes ont mal connus. L Castel 1843 [Google Scholar]
- Marshall JC, Halligan PW. Blindsight and insight in visuo-spatial neglect. Nature. 1988;336(6201):766–767. doi: 10.1038/336766a0. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379(6566):649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Mcdonald S, Tate RL, Rigby J. Error types in Ideomotor Apraxia: A qualitative analysis. Brain and Cognition. 1994;25(2):250–270. doi: 10.1006/brcg.1994.1035. [DOI] [PubMed] [Google Scholar]
- Milberg W, Blumstein SE. Lexical decision and aphasia: Evidence for semantic processing. Brain and Language. 1981;14(2):371–385. doi: 10.1016/0093-934x(81)90086-9. [DOI] [PubMed] [Google Scholar]
- Mirman D. Growth Curve Analysis and Visualization Using R. Chapman & Hall/CRC; 2014. [Google Scholar]
- Mirman D, Britt AE. What we talk about when we talk about access deficits. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1634) doi: 10.1098/rstb.2012.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Britt A, Cho PW. Aggregating fixation data across trials of different durations. 2012 Retrieved from http://www.danmirman.org/pdfs/LCDL-TR2012.04_Aggregation.pdf.
- Mirman D, Graziano KM. Damage to temporo-parietal cortex decreases incidental activation of thematic relations during spoken word comprehension. Neuropsychologia. 2012;50(8):1990–1997. doi: 10.1016/j.neuropsychologia.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Yee E, Blumstein SE, Magnuson JS. Theories of spoken word recognition deficits in Aphasia: Evidence from eye-tracking and computational modeling. Brain and Language. 2011;117(2):53–68. doi: 10.1016/j.bandl.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Magnuson JS. Dynamics of activation of semantically similar concepts during spoken word recognition. Memory & Cognition. 2009;37:1026–1039. doi: 10.3758/MC.37.7.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizelle JC, Wheaton Lewis A. The neuroscience of storing and molding tool action concepts: how “plastic” is grounded cognition? Frontiers in psychology. 2010;1:195. doi: 10.3389/fpsyg.2010.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlaas J. Contribution à l’étude de l’apraxie. Paris: Legrand. Legrand; Paris: 1928. [Google Scholar]
- Myung J, Blumstein SE, Sedivy JC. Playing on the typewriter, typing on the piano: manipulation knowledge of objects. Cognition. 2006;98(3):223–243. doi: 10.1016/j.cognition.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Myung J, Blumstein SE, Yee E, Sedivy JC, Thompson-Schill SL, Buxbaum LJ. Impaired access to manipulation features in Apraxia: Evidence from eyetracking and semantic judgment tasks. Brain and Language. 2010;112(2):101–112. doi: 10.1016/j.bandl.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarry C, Osiurak F, Delafuys D, Chauviré V, Etcharry-Bouyx F, Le Gall D. Apraxia of tool use: More evidence for the technical reasoning hypothesis. Cortex. 2013;49(9):2322–33. doi: 10.1016/j.cortex.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Peeters RR, Rizzolatti G, Orban GA. Functional properties of the left parietal tool use region. Neuroimage. 2013;78C:83–93. doi: 10.1016/j.neuroimage.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Pelgrims B, Olivier E, Andres M. Dissociation between manipulation and conceptual knowledge of object use in the supramarginalis gyrus. Human Brain Mapping. 2011;32(11):1802–1810. doi: 10.1002/hbm.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poizner H, Clark M, Merians AS, Macauley B, Rothi LJG, Heilman KM. Joint coordination deficits in limb Apraxia. Brain. 1995;118(1):227–242. doi: 10.1093/brain/118.1.227. [DOI] [PubMed] [Google Scholar]
- Poppel E, Held R, Frost D. Residual visual function after brain wounds involving the central visual pathways in man. Nature. 1973;243(5405):295–296. doi: 10.1038/243295a0. [DOI] [PubMed] [Google Scholar]
- Puce A, Perrett D. Electrophysiology and brain imaging of biological motion. Philosophical Transactions of the Royal Society B. 2003;358(1431):435–445. doi: 10.1098/rstb.2002.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nature Reviews Neuroscience. 2005;6(7):576–582. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Rafal R, Ward R, Danziger S. Selection for action and selection for awareness: evidence from hemispatial neglect. Brain Research. 2006;1080(1):2–8. doi: 10.1016/j.brainres.2005.01.108. [DOI] [PubMed] [Google Scholar]
- Randerath J, Goldenberg G, Spijkers W, Li Y, Hermsdörfer J. Different left brain regions are essential for grasping a tool compared with its subsequent use. Neuroimage. 2010;53(1):171–180. doi: 10.1016/j.neuroimage.2010.06.038. [DOI] [PubMed] [Google Scholar]
- Randerath J, Li Y, Goldenberg G, Hermsdörfer J. Grasping tools: Effects of task and apraxia. Neuropsychologia. 2009;47(2):497–505. doi: 10.1016/j.neuropsychologia.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Renzi ED, Lucchelli F. Ideational Apraxia. Brain. 1988;111(5):1173–1185. doi: 10.1093/brain/111.5.1173. [DOI] [PubMed] [Google Scholar]
- Riddoch MJ, Riveros R, Humphreys GW. Functional relations trump implied motion in recovery from extinction: Evidence from the effects of animacy on extinction. Neurocase. 2011;17(1):1–10. doi: 10.1080/13554791003785919. [DOI] [PubMed] [Google Scholar]
- Roberts KL, Humphreys GW. Action relations facilitate the identification of briefly-presented objects. Attention, Perception, & Psychophysics. 2011;73(2):597–612. doi: 10.3758/s13414-010-0043-0. [DOI] [PubMed] [Google Scholar]
- Rothi LJG, Heilman KM. Ideomotor apraxia: Gestural learning and memory. In: Roy EA, editor. Neuropsychological studies in apraxia and related disorders. New York: Oxford University Press; 1985. pp. 65–74. [Google Scholar]
- Sakreida K, Scorolli C, Menz MM, Heim S, Borghi AM. Are abstract action words embodied? An fMRI investigation at the interface between language and motor cognition. Frontiers in Human Neuroscience. 2013;7:125. doi: 10.3389/fnhum.2013.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL. Localizing interference during naming: Convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(1):322–327. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuil KDI, Smits M, Zwaan RA. Sentential context modulates the involvement of the motor cortex in action language processing: an fMRI study. Frontiers in Human Neuroscience. 2013;7:100. doi: 10.3389/fnhum.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Friston KJ, O’Doherty J, Wolpert DM, Frith CD. Activation in posterior superior Temporal sulcus parallels parameter inducing the percept of animacy. Neuron. 2005;45(4):625–635. doi: 10.1016/j.neuron.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Brecher AR, Whyte J, Klein MG. A patient registry for cognitive rehabilitation research: A strategy for balancing patients’ privacy rights with researchers’ need for access. Archives of Physical Medicine and Rehabilitation. 2005;86(9):1807–1814. doi: 10.1016/j.apmr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specialization within the semantic system. Cognitive Neuropsychology. 1988;5:133–142. [Google Scholar]
- Sirigu A, Cohen L, Duhamel JR, Pillon B, Dubois B, Agid Y. A selective impairment of hand posture for object utilization in apraxia. Cortex. 1995;31:41–55. doi: 10.1016/s0010-9452(13)80104-9. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y. The mental representation of hand movements after Parietal cortex damage. Science. 1996;273(5281):1564–1568. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- Smania N, Aglioti SM, Girardi F, Tinazzi M, Fiaschi A, Cosentino A, Corato E. Rehabilitation of limb apraxia improves daily life activities in patients with stroke. Neurology. 2006;67(11):2050–2052. doi: 10.1212/01.wnl.0000247279.63483.1f. [DOI] [PubMed] [Google Scholar]
- Smania N, Girardi F, Domenicali C, Lora E, Aglioti S. The rehabilitation of limb apraxia: a study in left-brain-damaged patients. Archives of Physical Medicine and Rehabilitation. 2000;81(4):379–388. doi: 10.1053/mr.2000.6921. [DOI] [PubMed] [Google Scholar]
- Springer A, Huttenlocher A, Prinz W. Language-induced modulation during the prediction of others’ actions. Psychological Research. 2012;76(4):456–466. doi: 10.1007/s00426-012-0411-6. [DOI] [PubMed] [Google Scholar]
- Stamenova V, Roy EA, Black SE. Associations and dissociations of transitive and intransitive gestures in left and right hemisphere stroke patients. Brain and Cognition. 2010;72(3):483–490. doi: 10.1016/j.bandc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Buccino G, Saccuman MC, Gallese V, Danna M, Scifo P, Fazio F, Rizzolatti G, Cappa SF, Perani D. Listening to action-related sentences activates fronto-parietal motor Circuits. Journal of Cognitive Neuroscience. 2011;17(2):273–281. doi: 10.1162/0898929053124965. [DOI] [PubMed] [Google Scholar]
- Tidoni E, Borgomaneri S, Pellegrino G di, Avenanti A. Action simulation plays a critical role in deceptive action recognition. Journal of Neuroscience. 2013;33(2):611–623. doi: 10.1523/JNEUROSCI.2228-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper SP, Paul MA, Hayes AE. Vision-for-action: The effects of object property discrimination and action state on affordance compatibility effects. Psychonomic Bulletin & Review. 2006;13(3):493–498. doi: 10.3758/bf03193875. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Ostrin RK. The processing of simple and complex words in an agrammatic patient: Evidence from priming. Neuropsychologia. 1994;32(8):1001–1013. doi: 10.1016/0028-3932(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Vanbellingen T, Bohlhalter S. Apraxia in neurorehabilitation: Classification, assessment, and treatment. Neurorehabilitation. 2011;28:91–98. doi: 10.3233/NRE-2011-0637. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, Acke F, Vandemaele P, Achten E. Tool responsive regions in the posterior parietal cortex: effect of differences in motor goal and target object during imagined transitive movements. Neuroimage. 2009;47(4):1832–1843. doi: 10.1016/j.neuroimage.2009.05.100. [DOI] [PubMed] [Google Scholar]
- Warrington EK, McCarthy RA. Categories of knowledge: further fractionations and an attempted integration. Brain. 1987;110:1273–1296. doi: 10.1093/brain/110.5.1273. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107:829–853. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Warrington Elizabeth K, Shallice T. Semantic access Dyslexia. Brain. 1979;102(1):43–63. doi: 10.1093/brain/102.1.43. [DOI] [PubMed] [Google Scholar]
- Watson CE, Cardillo ER, Ianni GR, Chatterjee A. Action concepts in the Brain: An activation likelihood estimation meta-analysis. Journal of Cognitive Neuroscience. 2013;25(8):1191–205. doi: 10.1162/jocn_a_00401. [DOI] [PubMed] [Google Scholar]
- Witt JK, Kemmerer D, Linkenauger SA, Culham J. A functional role for motor simulation in identifying tools. Psychological science. 2010;21(9):1215–1219. doi: 10.1177/0956797610378307. [DOI] [PubMed] [Google Scholar]
- Wu DH, Waller S, Chatterjee A. The functional neuroanatomy of thematic role and locative relational knowledge. Journal of Cognitive Neuroscience. 2007;19(9):1542–1555. doi: 10.1162/jocn.2007.19.9.1542. [DOI] [PubMed] [Google Scholar]
- Yee E, Chrysikou EG, Hoffman E, Thompson-Schill SL. Manual experience shapes object representations. Psychological Science. 2013;24:909–919. doi: 10.1177/0956797612464658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee E, Blumstein SE, Sedivy JC. Lexical-Semantic Activation in Broca’s and Wernicke’s Aphasia: Evidence from Eye Movements. Journal of Cognitive Neuroscience. 2007;20(4):592–612. doi: 10.1162/jocn.2008.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee E, Sedivy JC. Eye movements to pictures reveal transient semantic activation during spoken word recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32(1):1–14. doi: 10.1037/0278-7393.32.1.1. [DOI] [PubMed] [Google Scholar]
- Zwinkels A, Geusgens C, van de Sande P, van Heugten C. Assessment of apraxia: inter-rater reliability of a new apraxia test, association between apraxia and other cognitive deficits and prevalence of apraxia in a rehabilitation setting. Clinical Rehabilitation. 2004;18(7):819–827. doi: 10.1191/0269215504cr816oa. [DOI] [PubMed] [Google Scholar]