Abstract
Background
Resection of motor pathway gliomas requires the intraoperative recognition of essential cortical-subcortical motor structures. The degree of involvement of motor structures is variable, and increases as result of treatments patients are submitted to. Intraoperative neurophysiology offers various stimulation modalities, which efficiency is based on the ability to recognize essential sites with the highest possible resolution in most clinical conditions. Two stimulation paradigms evolved for intraoperative guidance of motor tumors removal: the 60 Hz-technique [low frequency (LF)] and the pulse-technique [high frequency-(HF)], delivered by bipolar or monopolar probe respectively. Most surgical teams rely on to either of the 2 techniques. The key point is the integration of the choice of the stimulation modality with the clinical context.
Methods
In 591 tumors involving the corticospinal tract, the use of HF and LF was tailored to the clinical context defined by patient clinical history and tumor features (by imaging). The effect was evaluated on the feasibility of mapping, the impact on immediate and permanent morbidity, the extent of resection, and the number of patients treated.
Results
By integrating the choice of the probe and the stimulation protocol with patient clinical history and tumor characteristics, the best probe-frequency match was identified for the different sets of clinical conditions. This integrative approach allows increasing the extent of resection and patient functional integrity, and greatly expands the number of patients who could benefit from surgery.
Conclusions
The integration of stimulation modalities with clinical context enhances the extent and safety of resection and expands the population of patients who could benefit from surgical treatment.
Keywords: brain mapping, extent of resection, gliomas, intraoperative neurophysiology, motor pathways, outcome
The role of surgery in the treatment of gliomas is crucial for relieving symptoms and determining histological and molecular diagnosis. The main oncological endpoints for surgery, such as progression-free survival, overall survival and, in low-grade gliomas, malignant progression-free survival, greatly depend on the extent of surgical resection.1 The factors limiting the extent of tumor resection are the highly infiltrative nature of gliomas and their frequent localization in the so-called “eloquent areas,” the areas considered essential in the neural networks underlying complex functions such as sensory-motor, language, or visuospatial activities.2–4 A lesion of an eloquent area results in a neurological deficit; therefore, in modern surgery it is mandatory that maximum tumor resection be accomplished without damage to the patient's functional integrity.3,4 Accordingly, the surgical approach has been changed from the traditional one, which relied on anatomical references, to a new one aimed at recognizing the different neural structures based on their functional properties with the aid of intraoperative neurophysiology.4 The most critical issue is identifying, with use of the appropriate neurophysiological techniques during surgery, the eloquent essential areas to be preserved. To this aim, electrophysiological investigation is supported by preoperative neuroradiological studies to provide the surgeon with a high-quality anatomical frame for guiding functional resection.5 This multidisciplinary approach allows the modern neurosurgeon to increase the number of cases treated by surgery.
Resecting tumors involving the motor pathways is critical, particularly those of the corticospinal system, given their very low degree of postoperative plasticity (ie, the ability to transfer their function to another network nearby or distant from the original network).6 The ability to track functional structures at cortical and subcortical levels, where the surgeon faces systems of fibers of indefinite origin, strictly depends on the protocol of stimulation. Two techniques have been developed to assess the motor pathways during surgery: the recent “train-of-five” technique (To5) and the traditional 60 Hz technique.7 The To5 technique delivers short (10–18 millisecond) trains of high-frequency (250–500 Hz) square wave, monophasic pulses with a low-train repetition rate (0.5–2 Hz), while the 60 Hz technique delivers long trains (1–4 s) of biphasic pulses at low frequency (60 Hz). Most surgical teams rely onto either of the 2 techniques without combining them during surgery, although an integrative approach may increase the efficiency of mapping. To date, few groups have reported comparative studies on the different stimulation techniques adopted for motor mapping, and no data are available on their clinical results.7,8 The key point is integrating the choice of stimulation modality with the clinical context. Gliomas are highly heterogeneous tumors, and their degree of heterogeneity increases as a result of the treatments the patients receive. Regarding tumors within the motor pathways, the degree of involvement of the corticospinal tract (CST) is generally quite variable, which determines the various spectra of patient clinical history and presentation and influences the level of excitability of the motor pathways at surgery. This can be predicted by looking at clinical parameters such as the history of seizures and their control, the number and doses of antiepileptic drugs (AEDs), the history of previous treatments, and the neurological status of the patient. In addition, the appearance of the tumor on conventional volumetric fluid-attenuated inversion recovery (FLAIR) images or preoperative diffusion tensor imaging (DTI) reconstruction of the CST may provide the surgeon with the view of motor pathways involvement and represent a useful tool for planning the surgical and neurophysiological strategy and defining the chance of resection.5,9
This study reports the data obtained from the combined use of high frequency (HF) and low frequency (LF) for surgical removal of many gliomas involving the motor pathways. Our data demonstrate that the combination of the 2 techniques, as tailored to the clinical context and defined by patient clinical history and tumor features shown by imaging, increases the reliability of mapping, expands the number of patients who could benefit from surgery, optimizes the extent of resection, and decreases permanent morbidity.
Materials and Methods
Patients
Five-hundred ninety-one patients with gliomas within the motor pathways, operated from 2007 to 2012, were included and gave written informed consent for the surgical and mapping procedure, which followed the principles outlined in the World Medical Association Declaration of Helsinki statement on ethical principles of research involving human subjects. Demographic and clinical features at admission were documented, and particular attention was given to history of seizures (number and duration), number and doses of AEDs, seizure control, history of previous treatments (surgery, chemotherapy, radiotherapy), and neurological status. Patients underwent preoperative neuropsychological evaluation, baseline MR studies, functional MR imaging (fMRI), and DTI with fiber tractography (DTI-FT). Volumetric scan analysis was used for defining tumor location and volume.10 Tumor volume was computed on volumetric FLAIR MRI scans for low-grade gliomas (LGGs) and on postcontrast T1-weighted MRI scans for high-grade gliomas (HGGs).10–12 All patients underwent intraoperative neurophysiological brain mapping and monitoring. Histology was classified according to the WHO brain tumor classification.
Surgical Procedure
A craniotomy, tailored to expose the cortex corresponding to the tumor area and a limited amount of surrounding tissue, was performed. Cortical mapping was performed to define the cortical safe-entry zone. Subcortical brain mapping was then continued, along with tumor resection. For prerolandic tumors located in the proximity of the CST, resection was started from the posterior border where the CST was located and, after its identification, the tract was followed inside the tumor mass. Afterwards, the remaining anterior part of the tumor was removed. Similarly, for parietal tumors, resection was started from the anterior border, following the same principle. For rolandic tumors, the peripheral functional motor borders were initially located all around the tumor, and then the tumor mass was removed. In all cases, resection followed the principle of locating the functional motor or language tracts, which represented the limits of resection.
Neurophysiological Brain Monitoring
Neurophysiological monitoring involved simultaneous acquisition of continuous electroencephalography (EEG), electrocorticography (ECoG), multichannel recording of free-running electromyographic (EMG) activity, and motor-evoked potentials (MEPs) (Comet-EEG-system, Grass-Astro-Med, ISIS-IOM, and InomedGmbH).
EEG was recorded bilaterally by 2 subdermal needle electrodes in the frontal and postcentral parietal regions of each hemisphere, providing 4 bipolar (coupled in 2 longitudinal and 2 transversal leads) or/and monopolar (each electrode referred to a midfrontal electrode) derivations. The ongoing EEG signal provided a real-time monitor of the depth of anesthesia and the possible occurrence of seizures involving brain areas distal to those that were exposed. EEG was recorded from the beginning of anesthesia in parallel with ECoG throughout surgery.
ECoG was recorded by a subdural strip electrode with 4–6 contacts (Cortical-Strip-Electrode, Integra) in a monopolar array referred to the midfrontal electrode and inserted close to the region to be mapped. The EcoG signal provided a real-time monitor for depth of anesthesia and possible occurrence of afterdischarges during stimulation and/or focal seizures throughout resection. EEG and EcoG signals were filtered (bandpass 1–100 Hz), displayed with high sensitivity (50–150 μm/cm and 300–500 μm/cm respectively), and recorded.
To monitor the integrity of descending motor pathways throughout the procedure, To5 stimulation was delivered to the primary motor cortex to elicit MEPs.13,14 To this aim, a 4-contact subdural strip electrode was placed over the precentral gyrus. Each contact was tested with a vertex reference by stimulation with trains of 3–5 constant current anodal pulses (pulse duration 0.5–0.8 milliseconds; interstimulus interval [ISI] 2–4 milliseconds) at 1 Hz. The motor threshold (MT) corresponded to the lowest intensity allowing a reproducible MEP (peak-to-peak amplitude >0.2 mV) in a distal muscle. The contact showing the lowest MT was chosen for monitoring, and the optimal ISI was determined for every patient. The default pulse duration was 0.5 milliseconds, increased to 0.8 milliseconds in patients with high MT. The train repetition rate was increased up to 1.5 Hz during critical phases of surgery (required by the neurosurgeon). MEPs were recorded by pairs of hooked subdermal needle electrodes (Technomed) placed in a muscle-tendon array, and target muscles for monitoring were chosen according to cortical exposure (in upper limb: extensor digitorum communis, abductor digiti minimi, first dorsal interosseus, abductor pollicis brevis; in lower limb: tibialis anterior, triceps surae, flexor hallucis brevis) (for example see Fig. 2). During surgery, MEPs were continuously monitored in asleep and in awake patients and suspended only during cortical and subcortical mapping to avoid interference. The background muscle activity was also monitored from the very beginning of surgery by multiple EMG recordings. Up to 24 muscles were recorded from the head to the foot contralateral to the operated brain hemisphere, along with a few recordings on ipsilateral muscles. EMG activity was collected, filtered, and displayed by the same equipment used for MEP monitoring (ISIS IOM Inomed). Free-running EMG was used to record responses to stimulation and to distinguish between electrical and clinical seizures. Electrical seizures are detected on ECoG only; in clinical seizures, ECoG alteration is accompanied by ictal muscle activation detected on free-running EMG.15
Fig. 2.
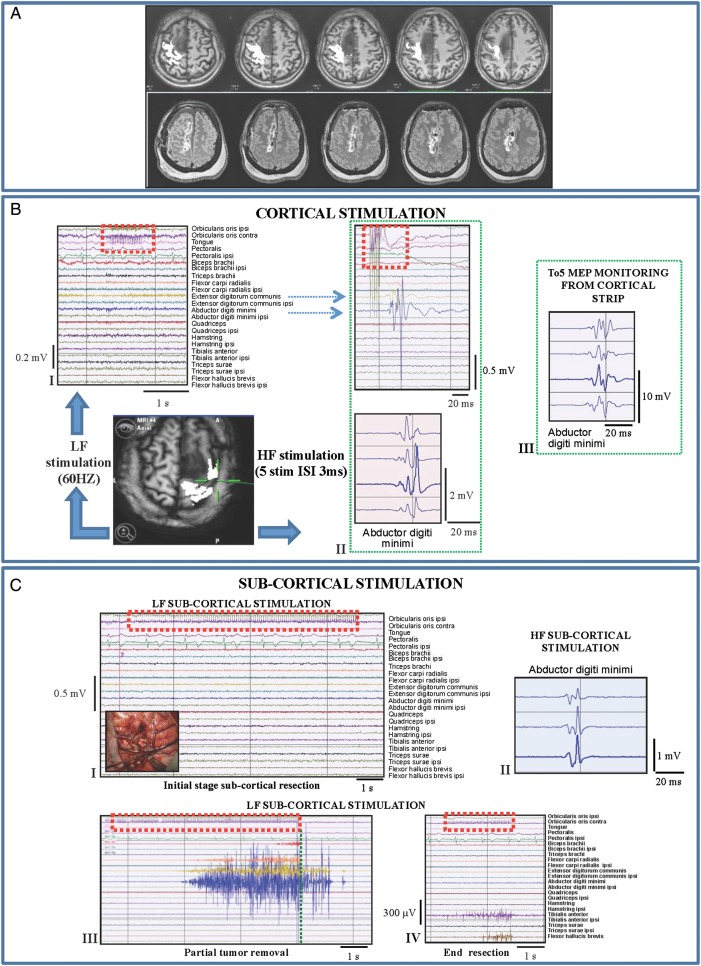
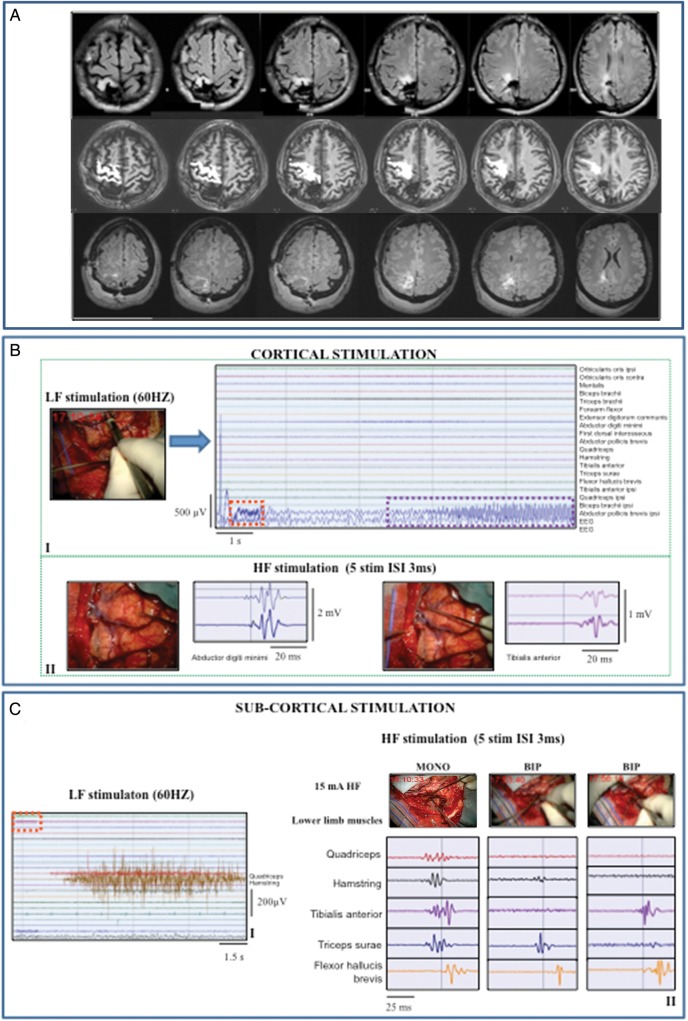
Combined low-frequency (LF; 60 Hz)/high-frequency (HF) stimulation: tumor highly displacing and infiltrating the CST (Group 1/1subgroup BI). Representative case: a 38-year-old female with an 8-month history of partial motor seizures, followed in the recent past by some generalized seizures controlled by the administration of 2 AEDs. MR studies showed a right (nondominant) prerolandic mass resembling a low-grade glioma. She was operated under asleep anesthesia. Histology revealed a grade II oligodendroglioma, IDH1 mutated, MGMT methylated, codeleted. Immediate postoperative course was characterized by left motor weakness (3/5), lasting 10 days and fully recovered. (A) Preoperative (DTI-FT reconstructionions of CST, in white, are superimposed on T1-weighted images) and postoperative MRI scan (FLAIR images) (Group 1) of the representative case. The CST was displaced and highly infiltrated by the tumor. Postoperative MR (FLAIR) showed complete resection. (B) (I) Failure of LF stimulation (bipolar probe) over the primary motor cortex with intensity up to 7 mA (stimulus artifact indicated by dashed red line). (II) HF stimulation (train of 5 anodal square pulses, 0.5 milliseconds duration, ISI 3 milliseconds, 1 Hz repetition rate) of the primary motor cortex with a monopolar probe evoked a motor response in hand/forearm muscles. MEPs are displayed on the monitor with all the recorded muscles (EMG free-running modality, upper window) and in a separate monitor in triggered modality (lower window) allowing display of single muscles for more precise monitoring. Both LF (I) and HF (II) stimulation were applied over the same site indicated by the intraoperative screen shot (DTI-FT reconstruction of CST, in white, superimposed on T1-weighted images). (III) Continuous MEP monitoring in abductor digiti minimi from the cortical strip (5 anodal square pulses, 0.5 milliseconds duration, 3 milliseconds ISI, 1 Hz repetition rate). (C) (I) Failure of the LF at initial stage subcortical resection (8 mA) (the intraoperative picture shows the site of stimulation). Over the same subcortical site, HF (II) successfully evoked response in hand muscle (16 mA monopolar probe). (III) After partial tumor removal, LF stimulation of the CST evoked response in hand/forearm muscles (7 mA). The activated muscles show a sustained clonic motor activity, continuing 1.736 milliseconds beyond the end of the stimulus (vertical dashed green line) and stopped by cold irrigation of the stimulated site. (IV) At the end of resection, LF stimulation evoked an EMG activation in lower limb muscles (4 mA).
Neurophysiological Brain Mapping
Two stimulation techniques were used, low frequency (LF) and high frequency (HF), so called according to the frequency of pulses within the delivered trains.
The LF stimulation consisted in trains lasting from 1 to 4 seconds of biphasic square wave pulses (0.5 milliseconds each phase) at 60 Hz (ISI 16.6 milliseconds) delivered by a constant current stimulator (OSIRIS-NeuroStimulator) integrated into the ISIS-System or, in the historical group of patients (Group 2), by an Ojemann-Cortical-Stimulator (Integra) through a bipolar probe (2 balltips, 2 mm diameter, spaced by 5 mm). For the first trial of stimulation in study Group 1, during the mapping at cortical level, for the first trial of stimulation, LF was set to an initial intensity of 2 mA, then progressively increased in steps of 0.5 mA until a muscle response was clearly detected on the free-running EMG (cortical Low frequency motor threshold, [cLF-MT]). During subcortical mapping, LF stimulation was either alternated with resection in a back-and-forth fashion or coupled with resection by keeping the stimulation probe and instruments used for resection in the same position. The subcortical stimulation was initially set at the intensity adopted for cortical mapping; in Group 1 only, when a stable motor response was obtained, the intensity was decreased to the minimum value needed to evoke an EMG response (subcortical low frequency motor threshold, [sLF-MT]). LF mapping was considered ineffective in the absence of motor response with a stimulus intensity of 15 mA or when electrical or clinical seizures occurred at intensities <15 mA .
HF stimulation was delivered by a monopolar probe (straight tip, 1.5 mm diameter frontal reference; Inomed) and also by a bipolar probe in a selected group of patients. HF stimulation was delivered according to the same parameters used for the MEP monitoring procedure (3–5 pulses, pulse duration 0.5–0.8 milliseconds, ISI 2–4 milliseconds) with anodal polarity for cortical stimulation and cathodal polarity for subcortical stimulation. At cortical site the current intensity was initially set at the MT used for MEPs monitoring, then, during mapping, in each site eliciting motor responses, the current was adjusted to threshold (MEP peak-to-peak amplitude ≥ 0.1 mV, cortical high frequency motor threshold, cHF-MT) in each site eliciting motor responses. This protocol was repeated in all cortical sites of interest. During subcortical mapping, the stimulation -either alternated with resection or coupled with it- started with the same intensity adopted at cortical level and, when a stable motor response was obtained, the intensity was decreased to threshold (subcortical high- frequency motor threshold, [sHF-MT]) when a stable motor response was obtained. In a few patients showing high MTs with anodal stimulation, cathodal polarity allowed a lower MT for cortical stimulation and was also used for subcortical motor mapping. In patients harboring the lesion in the dominant hemisphere, intraoperative language mapping was also performed (Supplementary Materials).
Anesthesia
Total intravenous anesthesia was administered with propofol and remifentanil, and no muscle relaxants were employed during surgery to permit mapping of motor structures. Close attention was paid to prevent intraoperative seizures by carefully watching EcoG and free-running EMG. At the first ictal sign, stimulation was stopped, and cold irrigation was applied, which usually aborted the seizure. Whenever seizures spread to the whole hemibody, propofol bolus infusion (4 mL on average) was delivered.
Neuroimaging Study
Preoperative MR imaging was performed using a Philips Intera 3T scanner (Best). Standard MR evaluation for morphological characterization of lesions included axial T2-weighted TSE sequence (TR/TE 3000/85 milliseconds; field of view (FOV), 230 mm; 22 slices; section thickness, 5/1-mm gap; matrix, 512 × 512; SENSE factor, 1.5), axial 3D-FLAIR sequence (TR/TE 10 000/110 milliseconds; FOV, 230 mm; 120 slices; section thickness, 1.5/0-mm gap; matrix, 224 × 256; SENSE factor, 2) and postcontrast T1-weighted inversion recovery sequence (TR/TE 2000/10 milliseconds; FOV, 230 mm; 22 slices; section thickness, 5/1-mm gap; matrix, 400 × 512; SENSE factor, 1.5). Based on appearance in FLAIR images, tumor margins were reviewed by 2 experienced neuroradiologists (A.C. and A.F.) and classified as well defined when sharp borders were visible or diffuse. DTI data were obtained at 3T using a single-shot echo planar imaging (EPI) sequence (TR/TE 8986/80 milliseconds; b-value = 1 000 s/mm2; 32 diffusion gradients directions; FOV, 240; isotropic acquisition voxel dimensions, 2.5 × 2.5 × 2.5 mm; acquisition matrix, 96 × 96; 56 slices, no gap; SENSE factor, 2.5). An image set without diffusion weighting, b = 0 s/mm2, was also acquired. The data were interpolated in plane to a matrix of 256 × 256 leading to voxel size of 0.94 × 0.94 × 2.5 mm. Two consecutive acquisitions were obtained, and data were realigned and averaged offline to increase signal-to-noise ratio; thus, total time for DTI was ∼11 min. Axial 3D-FFE T1-weighted imaging (TR 8 milliseconds; TE 4 milliseconds; image resolution equal to DTI) was performed for anatomic guidance. Deterministic tractography was performed in all patients using DTI Studio v2.4.01 software (Jiang H, Mori S, Radiology Department, Johns Hopkins University) to obtain main eigenvector and fractional anisotropy (FA) maps. Subcortical connections were reconstructed using the fiber assignment by continuous tracking (FACT) method.16–18 An FA threshold of 0.1 and turning angle >55° were used as criteria to start and stop tracking.5,9 To reconstruct the component of the CST originating from M1, a region of interest was placed on an axial section at the level of subcortical white matter of the precentral gyri; eventual contaminating fibers were removed. 3D-T1-weighted or 3D-FLAIR images were coregistered to the mean of all diffusion-weighted images in order to superimpose white matter tracts on anatomical images, allowing comparison of the trajectories of tracts in the involved hemisphere with those of the contralateral unaffected hemisphere and evaluation of the anatomical relationship between the tract and the tumor mass and the effect exerted by the tumor on the tract of interest. DTI tractography images were systematically reviewed by 2 experienced neuroradiologists (A.C. and A.F.). Tracts were then classified as unchanged, displaced, or infiltrated/disrupted. Unchanged reconstructed tracts showed normal anisotropy, location, and orientation compared with homologous contralateral tracts. Displaced tracts had normal or only slightly decreased anisotropy and showed abnormal location or trajectories when compared with those of the contralateral unaffected hemisphere. Infiltrated tracts showed substantially decreased FA with abnormal hues on directional color maps because infiltrating tumor disrupts the directional organization of fiber tracts and causes altered color patterns on directional maps. In these cases, DTI tractography reconstructions superimposed on morphological series showed that tracts passed through the area of altered signal intensity on volumetric postcontrast T1-weighted or FLAIR images; they may still have a normal location if compared with contralateral unaffected tracts.5,9 The tractography data were saved in a compatible format (DICOM) using Medx Software (Medical Numerics).5 All sequences were coregistered offline and made available for intraoperative image guidance through a neuronavigation system (Brainlab). Lesion volumes were computed onto FLAIR volumetric sequences with manual segmentation using I PlanCranial software suite (BrainLab) by one investigator (M.R.). FLAIR hyperintense and T1W-gadolinium-enhanced signal abnormalities were included in the lesion load for LGGs and HGGs, respectively, and were then reported in cm3.
Postoperative Course
All patients were assessed for motor and language function within 1 week and at 1–3 months after surgery to detect acute deficits (occurring during the first week post surgery) and permanent deficits (observed at 3 months post surgery). Patients underwent both an immediate (within 48 h) and a 3-month postoperative MR scan (volumetric FLAIR and postGdT1-weighted images) to estimate the extent of resection (EOR). EOR corresponded to the percentage of the volume resected with respect to the preoperative volume: (preoperative volume-postoperative volume)/preoperative volume.1 The classification was here modified to include in the category of total resections, all the residual signal abnormalities smaller than 1 cm3 and to account for postoperative FLAIR changes related to edema and contusion of brain tissue surrounding the resection cavity due to surgical insult. Therefore, surgical outcome was categorized as (i) total resection (postoperative volume between 0–1 cm3), (ii) subtotal resection (postoperative volume between 1–10 cm3), and (iii) partial resection (postoperative volume >10 cm3).9 Postoperative diffusion-weighted MRI scans were also performed to check for ischemic damage.
Statistical Analysis
Data were analyzed using Prism 4 for Macintosh (GraphPad Software). Differences in the extent of resection were studied with the t test. Differences in percentage of clinical and electrical seizure and patient immediate and permanent deficits, as well as in the number of patients submitted for surgical treatment were studied with the Fisher' exact test.5
Results
Patients
On the basis of the neurophysiological procedure adopted during surgery, patients were divided into 2 groups. In Group 1 (479 patients) LF and HF were available and combined during mapping with the aid of complete EEG, ECoG, and To5-MEP monitoring. In this group of patients, we used the policy of starting motor mapping with LF being this stimulation modality the oldest and largely used by the neurosurgical community. In those cases in which LF was found ineffective (absence of a motor response with a stimulus intensity of 15 mA or when electrical or clinical seizures occurred at intensities <15 mA ), HF was applied for guiding resection. In Group 2, the historical group of the earliest 112 patients, only LF stimulation was available, and the monitoring setup included only EEG and EcoG without MEP monitoring. Group 2 represents the control group to be compared to Group 1.
Group 1: 479 patients (283 males males and 196 females, aged 17–58 years, median 37.4 years)
On a total of 479 tumors involving the motor pathways, 348 (72.7%) were LGGs, and 131 HGGs, progressed from low-grade gliomas to anaplastic form.
Group 2: 112 patients (55 males and 57 females, aged 19–64 years, median 41 years)
Of a total of 112 tumors, 75 (67%) were LGGs, and 37 HGGs, progressed from LGGs.
The detailed histological analysis and tumor location of both groups is shown in Table 1.
Table 1.
Tumor presentation in the 2 groups
| Group 1 (479 patients) |
Group 2 (112 patients) |
|||
|---|---|---|---|---|
| Histological diagnosis | Low-grade gliomas (348 patients, 72.7%) | 174 oligodendrogliomas | Low-grade gliomas (75 patients, 67%) | 48 oligodendrogliomas/ |
| 135 astrocytomas | 27 astrocytomas | |||
| 39 mixed low-grade gliomas | ||||
| High-grade gliomasa (131 patients, 27.3%) | 67 anaplastic oligodendrogliomas | High-grade gliomasa (37 patients, 33%) | ||
| 64 anaplastic astrocytomas | ||||
| Tumor location | 115 rolandic site | 32 rolandic site | ||
| 209 precentral site | 55 precentral site | |||
| 48 paralimbic site | 12 paralimbic site | |||
| 107 postcentral site | 13 postcentral site | |||
| DTI FT available | 389 patients (81.2%) | 44 patients (39.3%) | ||
aThese tumors were previously low-grade gliomas that progressed to anaplastic form.
Abbreviation: DTI-FT, diffusion tensor imaging with fiber tractography
Intraoperative Neurophysiological Mapping Results: Correlation with Clinical Data
Various subgroups were identified within Group 1 and Group 2, depending on the stimulating protocol adopted for mapping and the related clinical context (clinical history and imaging features on conventional MR and available DTI images).
Group 1 (combined HF+LF stimulation)
Within Group 1, two subgroups (subgroups A and B), were distinguished.
Subgroup A
In subgroup A (183 cases), the functional mapping was performed exclusively with LF stimulation (Table 2), which was successful in guiding resection at the cortical and subcortical levels. HF stimulation was used only to acquire data for comparison.
Table 2.
Subdivision of the experimental (group 1) and control (group 2) groups on the basis of mapping procedure
| Group 1 (479 patients) |
Group 2 (112 patients) |
|||
|---|---|---|---|---|
| Mapping procedure | Subgroup A: LF; HF only for comparison | Exclusively LF | ||
| Subgroup BI: HF to remove a significant portion of the tumor and to partially decompress CST; LF only for recognizing fibers belonging to the CST | ||||
| Subgroup BII: HF; LF (with HF) to complete the margin of resection | ||||
| Subgroup BIII: HF | ||||
| Subgroup BIV: HF (bipolar and monopolar probes) | ||||
| Number of patients | Subgroup A: 183 patients (38.2%) | Subgroup A: 68 patients (60.7%) | ||
| Subgroup B: 296 patients (61.8%) | Subgroup BI: 150 patients (50.6%) | Subgroup B: 44 patients (30.3%) | Subgroup BI: 36 patients (32.1%) | |
| Subgroup BII: 115 patients (38.8%) | ||||
| Subgroup BIII: 21 patients (7.2%) | Subgroup BII: 8 patients (7.2%) | |||
| Subgroup BIV: 10 patients (3.4%) | ||||
| Language mapping | 183 patients | 44 patients | ||
Abbreviations: DTI-FT, diffusion tensor imaging with fiber tractography; HF, high frequency; LF, low frequency
Clinical features.
Patients belonging to this subgroup were characterized by a short history of seizures that were well controlled by administration of 1–2 AEDs (Table 3). On admission, 177 patients were neurologically intact, and only 6 showed mild motor impairment.
Table 3.
Group 1: Patient clinical characteristics and tumor features defined by imaging
| Group 1 (479 patients) |
||||
|---|---|---|---|---|
| Subgroup A |
Subgroup B |
|||
| Clinical history: | ||||
|
171 patients (57.7%) received previous surgery, chemotherapy, and/or radiotherapy (8 patients radiotherapy only) | |||
| 3–6 months: 132 patients (72.1%) partial seizures only; 51 patients (27.9%) partial and generalized seizures | Median 6 seizures/month, either partial seizures only or partial seizures evolving into generalized seizures | |||
| One (82% of patients) or, less frequently, 2 | Poorly controlled by the administration of 2 to 4 AEDs, although prescribed at high doses | |||
| 177 (96.7%) intact, 6 (3.3%) mild motor impairment | 54 (18.2%) mild motor impairment (preoperative mild motor deficits in Subgroup BIII) | |||
| Tumor characteristics: | ||||
|
Well-defined mass with sharp borders | Tumor mass appeared diffuse with infiltrative (261 patients, 88.2%) | ||
| 79 precentral site | 130 precentral site | |||
| 21 paralimbic site | 27 paralimbic site | |||
| 51 postcentral site | 56 postcentral site | |||
| 32 rolandic site | 83 rolandic site (harboring tumors in a rolandic site, located within the CST: 6 cases in subgroup BIV) | |||
|
In 155 patients (84.7%) | Unaffected tract in 22.4% | In 234 patients (79%) | Highly displaced tract in 13.3% |
| Displacement of the tract's fibers in 39.9% | Highly displaced and infiltrated tract in 86.7% | |||
| Displacement and infiltration of the tract in 37.7% | ||||
| Histological diagnosis | Low-grade gliomas (126 patients, 68.9%) | Low-grade gliomas (222 patients, 75%) | ||
| High-grade gliomas (57 patients, 31.1%) | High-grade gliomas (74 patients, 25%) | |||
Abbreviations: AEDs, antiepileptic drugs; DTI-FT, diffusion tensor imaging with fiber tractography
Radiology.
The tumors appeared as well-defined masses with sharp borders in FLAIR-weighted MR images. Tumors were localized at rolandic (32), precentral (79), paralimbic (21), and postcentral (51) sites. DTI FT reconstruction of the CST, available in 155 cases (84.7%), showed an unaffected tract in 22.4% of cases, a displacement of the tract's fibers in 39.9%, and displacement and limited infiltration of the tract in 37.7% (Table 3).
Histology.
One-hundred twenty-six cases were LGGs, and 57 were HGGs. In 71 cases (38.8%), the tumor was recurrent (57 LGGs recurred as HGGs).
Mapping procedure.
LF cortical mapping was performed at threshold intensities (cLF-MT, Methods) ranging from 3 to 9 mA, always effective over the primary motor area (Fig. 1, Panel-B). Subcortical resection was continued until a threshold intensity (sLF-MT) ranging 2 to 3 mA was reached and was successful in all cases (Fig. 1, Panel-C).
Fig. 1.
Low-frequency (LF) stimulation (60 Hz ): tumors with sharp borders (Group 1/subgroup A †and Group 2/subgroup A). Representative case: 34-year-old female with a short history of partial seizures (2 episodes of speech arrest), fully controlled by administration of one AED. MR studies showed a left (dominant) prerolandic mass resembling a low-grade glioma. She was operated under asleep-awake anesthesia for motor and language mapping. Histology documented a grade II oligodendroglioma, IDH1 mutated, MGMT methylated, codeleted. In the immediate postoperative course, she experienced transient motor (4/5) deficits, mainly at the upper limb, fully recovered. (A) Preoperative (DTI-FT reconstructions of CST, in white, were superimposed on T1-weighted images) and postoperative (postcontrast T1-weighted) MRI scan (Group 1) of the representative case. The CST was mainly displaced and slightly infiltrated. The postoperative MR showed complete resection. (B) LF stimulation of the primary motor cortex with bipolar probe-evoked EMG responses (screen shot) in distal muscles of the contralateral forearm/hand. Stimulus intensity (no stimulus artifact is visible) = 2 mA. (C) LF stimulation of the subcortical CST with bipolar probe-evoked EMG responses in different muscle groups of upper limb. Stimulation at deeper portion of CST tract (III) also involved the lower limb muscles. †Stimulus intensity = 10 mA (I and II); 6 mA (III). Stimulus artifact indicated by dashed red line.
Seizures.
During the procedure, there were 61 (33.3%) electrical seizures and 31 overt clinical partial seizures (16.9%), which were aborted in 3–5 seconds by cold irrigation in most cases. Only 4 cases evolved into convulsive seizure requiring intravenous administration of propofol.
MEP monitoring.
MEP monitoring was available in all patients and documented a moderate (<50%) transient reduction of MEP in 16% of cases, which did not interfere with subcortical resection.
Subgroup B
In subgroup B (296 patients, Table 2) the combined use of LF and HF stimulation was required for performing and effectively mapping CST during resection.
Clinical features.
One-hundred seventy-one patients (57.7%) had undergone previous surgeries and chemotherapy and/or radiotherapy. Patients reported frequent seizures (median 6 seizures/month) that were poorly controlled by high doses of 2–4 AEDs, and 54 patients showed mild motor impairment on admission (Table 3).
Radiology.
In the majority of cases (261 patients), the tumors appeared diffuse and characterized by infiltrative borders on FLAIR images. DTI FT reconstruction of CST, available in 234 cases (79%), showed a highly displaced tract in 31 cases (13.3%) and a highly displaced and markedly infiltrated tract in 203 cases (86.7%). Tumors were located at rolandic (83), precentral (130), paralimbic (27), and postcentral (56) sites (Table 3).
Histology.
Two-hundred twenty-two cases (75%) were LGGs, and 74 were HGGs.
Mapping procedure.
Four different neurophysiological conditions were observed intraoperatively, clustering patients into 4 different subsets.
In the first subset of patients (1subgroup BI; 150 cases), intraoperative mapping required combined use of LF and HF stimulation. At the beginning, LF stimulation, although at high intensity, failed to elicit motor responses (Fig. 2, Panel-BI) on prerolandic cortical sites showing motor activation on fMRI and motor fibers on DTI-FT images. When applied over the same sites, the HF stimulation elicited responses (cHF-MT intensity: 5–12 mA, Fig. 2, Panel-BII), which allowed cortical mapping. Similarly, at the beginning of the subcortical resection, HF stimulation was required (Fig. 2, Panels-CI/II), while LF was not inducing subcortically motor responses. However, when a significant portion of the tumor was removed and the CST was decompressed, LF turned out to excite the CST fibers identified by the DTI-FT and always responsive to HF stimulation (Fig. 2, Panels-CIII/IV). Conclusively, in this subset of patients, HF stimulation was always efficient during resection, while LF stimulation was reliable only at the final stage of subcortical resection when HF and LF stimulation were delivered at threshold intensity (sHF-MT and sLF-MT) corresponding to 2 mA and 3 mA, respectively. Clinically these patients were characterized by a long history of seizures, receiving 2–3 AEDs, and radiologically by diffuse tumor on FLAIR images and high displacement and infiltration of the CST in DTI reconstruction.
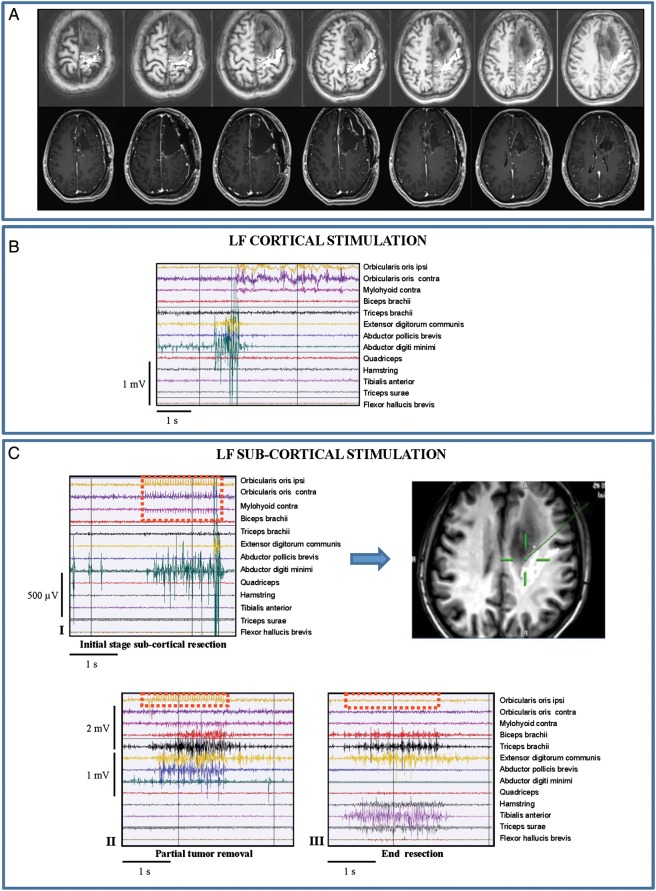
In the second subset (1subgroup BII; 115 cases), due to an altered cortical excitability, the LF cortical motor threshold intensity (cLF-MT: 2–6 mA) corresponded to the epileptic threshold (ie, the minimum current inducing motor responses with LF also triggered partial or even generalized seizures [rare]), which normally leads to cessation of LF stimulation (Fig. 3, Panel-BI). Conversely, HF stimulation (cHF-MT: 5–13 mA) allowed safe cortical mapping with no occurrences of seizures (Fig. 3, Panel-BII). HF stimulation was also needed during the main portion of subcortical resection when LF was not evoking motor responses or inducing seizures. LF elicited responses without seizures only at the final stage of resection when the CST had been largely decompressed; HF and LF were both used at threshold intensity (3 mA for HF and 5 mA for LF) to define the resection margins (Fig. 3, Panels-CI/II). Clinically, these patients were characterized by a long history of seizures that were poorly controlled by high doses of 2–4 AEDs. Radiologically, they presented with diffuse tumors on FLAIR images that highly infiltrated and displaced the CST in DTI reconstruction.
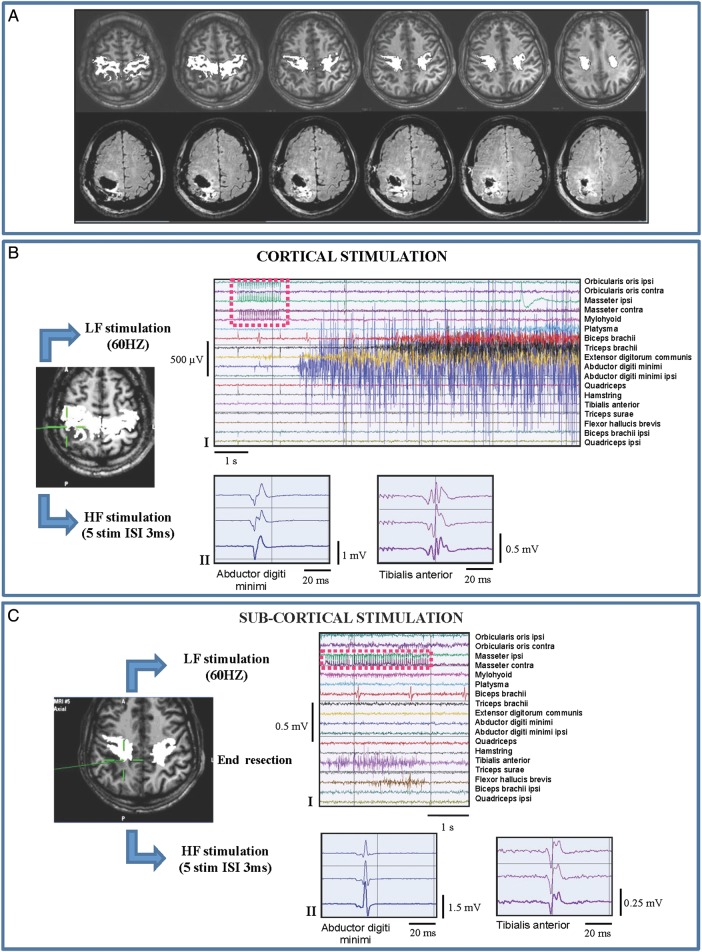
In the third subset of patients (1subgroup BIII; 21 cases), LF stimulation failed at both cortical and subcortical levels, (Fig. 4, Panels-BI-CI), even at the end of resection when high-current intensity elicited none or inconsistent small-amplitude EMG responses. Conversely, HF stimulation was always efficient (cHF-MT: 6–16 mA, sHF-MT: 3 mA), thus representing the unique tool to perform the functional mapping throughout resection (Fig. 4, Panels-BII-CII). Clinically, patients belonging to this subgroup were characterized by a long history of seizures, were taking 2–4 AEDs, and presented preoperatively with mild motor deficits. Radiologically they showed highly diffuse tumor masses with highly infiltrated CST on DTI images.
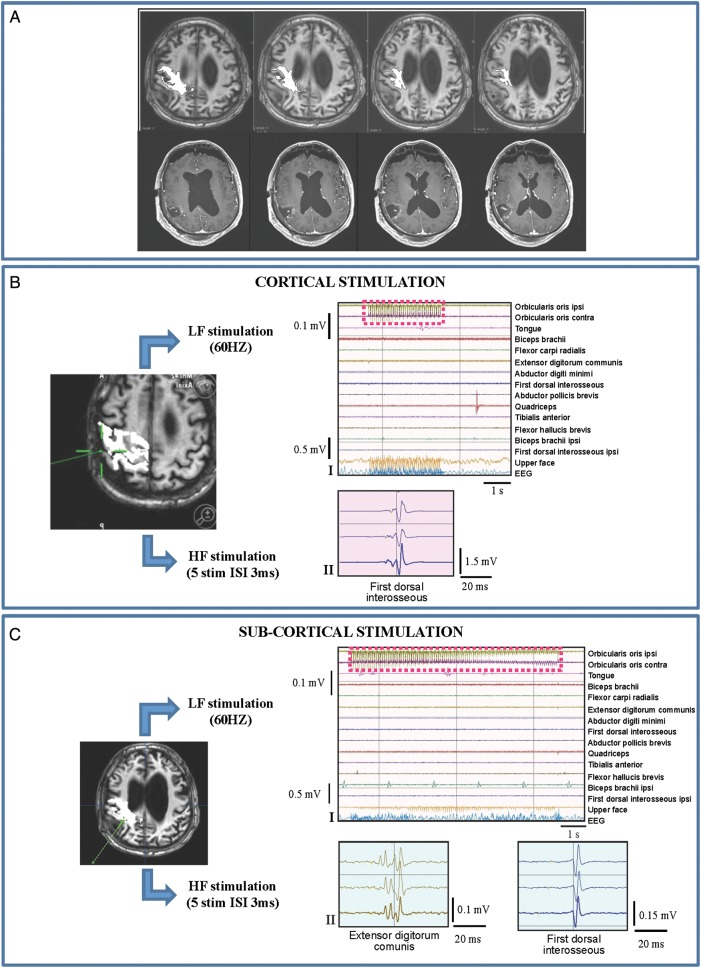
The fourth subset included patients (1subgroup BIV; 10 cases) with a long history of poorly controlled partial motor seizures who harbored rolandic tumors that were highly infiltrating or located within the CST. LF stimulation induced a high percentage of seizures (Fig. 5, Panels-BI/CI) and failed the mapping at both cortical and subcortical levels, while HF stimulation was always very efficient (Fig. 5, Panels-BII-CII). In this subset, HF was delivered with the monopolar and bipolar probes, both equally efficient, with some differences in spatial resolution (Fig. 5, Panel-CII). Specifically, the bipolar probe was used at the end of resection, because of its higher spatial resolution, to get closer to the CST and better define the margins of resection.
Fig. 3.
Combined low-frequency (LF; 60 Hz)/high-frequency (HF) stimulation: tumor highly displacing and infiltrating the CST and long history of seizures, poorly controlled by AEDs (Group 1/1subgroup BII). Representative case: a 41 year old male, with a 2 year history of motor partial seizures, increasing in frequency to 3–4 a week, associated in the recent past with several generalized seizures, poorly controlled by the administration of 4 AEDs at high dosage. MR studies showed a right (nondominant) postrolandic mass resembling a low-grade glioma. He was operated under asleep anesthesia. Histology revealed a grade II oligoastrocytoma, IDH1 wild-type, MGMT methylated, non-codeleted. Postoperative course was characterized by mild (4/5) motor weakness, recovered in 2 weeks. Seizures disappeared, and the number of AEDs reduced to one at 3 months post surgery. (A) Preoperative (DTI-FT reconstructions of CST, in white, are superimposed on T1-weighted images) and postoperative MRI scan (FLAIR images) (Group 1) of the representative case. The CST was highly infiltrated by tumor. Postoperative MR (FLAIR) showed a subtotal resection with the persistence of FLAIR abnormalities at the site of CST. (B) (I) LF stimulation (>10 mA, bipolar probe) over primary motor cortex evoked tiny response in forearm and hand muscles, but a seizure ensued starting from the muscles barely responding to stimulation and progressed to involve the whole upper limb. Stimulus artifact is indicated by red dashed line. The intraoperative screenshot (DTI-FT reconstruction of CST, in white, superimposed on T1-weighted images) indicates the site of stimulation. (II) Stimulation of the same site (screen shot) with HF stimulation (3–4 cathodal square pulses, 0.5 milliseconds duration, 3 milliseconds ISI, 1 Hz repetition rate, monopolar probe) at high intensity (21 mA) evoked MEPs in hand and lower limb muscles. (C) (I) At the end of surgery, a response in lower limb muscles was obtained by LF stimulation (4 mA). (II) In the final part of the resection, the motor threshold to HF stimulation was reduced to 8 mA. The intraoperative screenshot (DTI-FT reconstruction of CST, in white, superimposed on T1-weighted images) indicates the site of stimulation.
Fig. 4.
Combined low-frequency (LF; 60 Hz)/high-frequency (HF) stimulation: highly diffuse tumor mass, highly infiltrated CST, and preoperative mild motor deficits (Group 1/1subgroup BIII). Representative case: a 49-year-old-male with a 2-year history of motor partial seizures in the recent past and poorly controlled by administration of 4 AEDs. MR studies showed a right (nondominant) pre and postrolandic mass resembling a low-grade glioma and associated with ventricular enlargement (the patient had a previous history of intracerebral meningitis at birth). He was operated under asleep anesthesia. Histology revealed a grade II oligoastrocytoma, IDH1 nonmutated, MGMT methylated, non-codeleted. Immediate postoperative course was characterized by a mild motor weakness at the arm (4/5), recovered in one week. Seizures disappeared after surgery. (A) Preoperative (DTI-FT reconstruction of CST, in white, are superimposed on T1-weighted images) (Group 1) of the representative case. The CST was displaced and infiltrated by the tumor. Postoperative MR (T1) showed a partial resection, with the persistence of T1 abnormalities at the level of prerolandic and insular sites. (B) (I) Failure of LF stimulation over the primary motor cortex (8 mA, bipolar probe). Stimulus artifact is indicated by red dashed line. (II) HF stimulation (0.5 milliseconds duration, 4 milliseconds ISI, 1 Hz repetition rate, monopolar probe) evoked clear MEPs in the hand muscles (8 mA).The intraoperative screenshots (DTI-FT reconstructions of CST, in white, are superimposed on T1-weighted images) indicated the site of stimulation. (C) (I) Failure of LF stimulation at subcortical level (10 mA, bipolar probe). (II) HF stimulation (5 cathodal square pulses, 0.5 milliseconds duration, 4 milliseconds ISI, 1 Hz repetition rate, monopolar probe) evoked MEPs from the hand and the forearm muscles. The intraoperative screenshots (DTI-FT reconstructions of CST, in white, superimposed on T1-weighted images) indicated the site of stimulation.
Fig. 5.
Combined low-frequency (LF; 60 Hz)/high-frequency (HF) stimulation: tumor highly infiltrating or located within the CST and long history of poorly controlled partial motor seizures (Group 1/1subgroup BIV). Representative case: a 39-year-old male with a previous history of right postrolandic grade II oligodedroglioma, treated by surgery and followed by observation. Patient started to experience several partial sensory-motor seizures in the last 8 months, poorly controlled by AEDs (which were progressively increased in number and dosage). He was submitted to neuroradiological follow-up that showed a progressive increase of FLAIR abnormalities anterior to the previous surgical cavity within the CST, consistent with recurrence of the tumor. He was operated under asleep anesthesia. Histology showed a grade II oligodendroglioma, IDH1 mutated, MGMT methylated, codeleted. Immediate postoperative course was characterized by mild (4/5) weakness at the lower limb, fully recovered in one week. (A) Preoperative (FLAIR, upper), preoperative reconstruction of CST by DTI FT (white) superimposed on T1-weighted images (middle), and postoperative (FLAIR, lower) MRI scan of the representative case. Postoperative MR showed a subtotal resection with a little persistence (1,1 mL) of FLAIR abnormalities close to the lateral ventricle. (B) (I) LF stimulation of primary motor cortex (2 mA, bipolar probe) failed to evoke muscular responses (stimulus artifact indicated by red dashed square) while inducing electrical seizure on EcoG (violet dashed line). The intraoperative picture of the surgical field indicates the site of stimulation with the bipolar probe. (II) Over the same site, HF stimulation (5 anodal square pulses, 0.5 milliseconds duration, ISI 3 milliseconds, 1 Hz repetition rate, monopolar probe) induced MEPs without occurrence of seizures. The intraoperative picture of the surgical field indicated the site of stimulation with the monopolar probe. (C) (I) LF stimulation at subcortical level at the end of resection (7 mA, bipolar probe) failed to evoke muscular responses but triggered a focal seizure involving the lower limb muscles, which lasted 10.5 seconds and was interrupted by cold irrigation. (II) Subcortical mapping of the motor fibers with HF during surgical dissection. HF stimulation (cathodal square pulses, 0.5 milliseconds duration, ISI 3 milliseconds, 1 Hz repetition rate, 15 mA) was applied through monopolar and bipolar probes. Monopolar probe: activation of multiple muscles at the same site. Bipolar probe: focal responses of few muscles, which differed when the probe position was changed. The intraoperative picture of the surgical field indicated the site of stimulation with the monopolar or bipolar probes. MONO, monopolar probe; BIP, bipolar probe.
Seizures.
In subgroup B, 156 patients (52.7%) showed electrical seizures, specifically all patients in subgroup BII, 20 (13.2%) in subgroup BI, and 15 and 6 patients in subgroups BIII and BIV, respectively. Seizures occurred with LF stimulation, which triggered partial seizures in 39 paticipants (11%, mostly in subgroup BII). Only 6 patients (2%) developed clinical seizures during HF stimulation. The interruption of stimulation and use of cold irrigation controlled partial seizures. Only 3 cases (1%) spread to the whole hemibody and required infusion of propofol.
MEP monitoring.
MEP monitoring was available for all patients and documented moderate (<50%) transient reduction of MEP in 37.8% and severe transient reduction (>50%) in 7%. Moderate reduction of MEP did not interfere with subcortical resection, which was briefly interrupted or continued in distant areas of the surgical cavity and resumed after MEP normalization in cases of severe MEP reduction. Transient MEP loss was observed in only 2 cases. In those cases, MEP trace was recovered by increasing the current intensity (+2 mA) and increasing the level of blood pressure.
Group 2 (sole LF stimulation)
Two subgroups (subgroups A and B) were identified in the historical group (112 patients; Table 2) according to the neurophysiological findings observed intraoperatively and related clinical context.
Subgroup A
In the first subgroup (2Subgroup A; 68 cases), LF successfully allowed mapping in all cases at both the cortical and subcortical levels. The motor threshold intensity (cortical and subcortical LF-MT) was not assessed, and the same range of current intensity (3–8 mA) was used at the cortical and subcortical levels for guiding resection. Clinically, tumors were mostly LGGs, and patients free from neurological deficits reported a short history of seizures that were well controlled with one AED. Radiologically, patients presented with tumors with sharp margins in FLAIR images and DTI, when available, showed a displaced CTS with limited infiltration (Table 4). Twenty-five percent of the patients experienced intraoperative seizures.
Table 4.
Group 2: Patients clinical characteristics and tumor features defined by imaging
| Group 2 (112 patients) |
|||
|---|---|---|---|
| 2Subgroup A | 2Subgroup BI | 2Subgroup BII | |
| 68 patients (60.7%) | 36 patients (32.1%) | 8 patients (7.2%) | |
| Clinical history | |||
| Previous treatments | 16 (23.5%) | 19 (52.7%) | 2 (25%) |
| Seizures | Short history of seizures | Several seizures | Short history of seizures |
| AEDs | 1 | 2–3 | 1–2 |
| Neurological deficits | Free | 5 mild (13.9%) | 7 mild (87.5%) |
| Tumor characteristics | Sharp margins | Diffuse margins and edema | Large tumors with edema |
| DTI FT available | 22 patients (32.3%) displaced CTS and with a limited infiltration | 17 patients (47.2%) displaced and highly infiltrated CST | 5 patients (62.5%) Significantly dislocating and infiltrating the motor area and tract |
| Histological diagnosis | 54 LGG (79.4%) and 14 HGG (20.6%) | 20 LGG (55.6%) and 16 HGG (44.4%) | 8 HGG (progressive LGG) |
Abbreviations: AEDs, antiepileptic drugs; DTI-FT, diffusion tensor imaging with fiber tractography; HGG, high-grade glioma; LGG, low-grade glioma
Subgroup B
In subgroup B, LF stimulation was partially or totally inefficient at the cortical and/or subcortical level in 44 patients. Two subsets were identified according to the clinical and radiological scenario and associated intraoperative neurophysiological findings (Table 4).
In the first subset (2Subgroup BI; 36 cases), patients reported a long history of seizures, controlled with 2–3 AEDs, and 5 patients had preoperative mild to moderate motor impairment. Radiologically, these patients had tumors with diffuse margins or edema in FLAIR images and highly infiltrated CST in DTI images. Intraoperatively, the high intensity needed for LF at the cortical level (range 10–18 mA) to induce motor responses led to a significant occurrence of seizures (18 cases, 50%), generalized in 3 cases (8.3%). The subcortical mapping was performed by applying LF at current intensities higher (beginning) or equal (end of resection) to those applied over the cortex and occasionally led to seizures (4 cases, 11.1% partial).
The second subset (2Subgroup BII; 8 cases) clustered patients with large tumors and considerable edema dislocating/infiltrating the CST. Seven patients showed preoperative mild motor deficits. LF stimulation, despite the high intensity (average 20 mA), produced negative results while mapping over cortical areas reporting fMRI motor activation and/or DTI CST reconstruction. In these cases, resection was started in an area of tumor distant from the fMRI/DTI-positive sites. After a partial tumor removal, LF turned out to work, although with a high intensity (mean 15 mA), which was associated in 4 patients (50%) to partial seizures.
Impact of the Different Mapping Protocols on EOR and the Occurrence of Transient and Permanent Motor Deficits
The extent of resection (EOR) and the occurrence of transient and permanent deficits were assessed in all patients.
Group 1
Subgroup A (LF efficient, HF for comparison; LF-MT current used)
In a total of 183 procedures, EOR was estimated as total in 154 cases (84.2%), subtotal in 20 cases (10.9%), and partial in 9 (4.9%).
Acute motor deficits occurred in 109 patients (59.5%), fully recovered in all patients.
Subgroup B (LF–HF Combined; LF-MT and HF-MT Current Used)
1Subgroup BI (150 patients. HF used; LF inefficient and used at the end of resection).
EOR was total in 105 cases (70%) and subtotal in 45 (30%).
Acute motor deficits were observed in 103 patients (68.7%, including those with preoperative deficits) and permanent deficits in 4 patients (2.7%, including 2 patients with previous deficits).
1Subgroup BII (115 patients; HF used; LF induced seizures and was used at the end of resection). EOR was total in 67 cases (58.3%), subtotal in 41 (35.7%), and partial in 7 (6%).
Acute motor deficits occurred in 64 patients (55.6%) including those with preoperative deficits, and permanent deficits occurred in 2 patients (1.7%).
1Subgroup BIII (21 patients. HF used; LF totally inefficient).
EOR was subtotal in 12 (57.2%) cases and partial in 9 cases (42.8%).
Acute motor deficits occurred in 18 (85.7%) patients (including 16 with preoperative deficits), and permanent deficits occurred in 2 patients (9.5%).
1Subgroup BIV (10 patients. HF used, both probes tested; LF totally inefficient)
EOR was total in 6 (60%) cases and subtotal in 4 (40%).
Acute motor deficits occurred in 1 patient, who recovered fully.
Group 2
Subgroup A (LF Efficient. No LF-MT Current Assessed)
In 68 procedures, EOR was total in 49 (72.1%) cases and subtotal in 19 (27.9%).
Acute motor deficits occurred in 42 (61.8%) patients, who recovered fully.
Subgroup B (LF Partially or Not Successful)
2Subgroup BI (36 patients; LF induced seizures)
EOR was total in 21 (58.4%) cases, subtotal in 12 (33.3%) cases, and partial in 3 cases (8.3%).
Acute motor deficits occurred in 34 patients (94.4%, including those with preoperative deficits). Four (11.1%) patients had permanent deficits.
2Subgroup BII (8 patients. LF totally inefficient)
EOR was total in 5 (62.5%) cases and subtotal in 3 (37.5%) cases.
All (100%) patients showed acute motor deficits, and 6 (75%) had permanent deficits.
Clinical Remarks
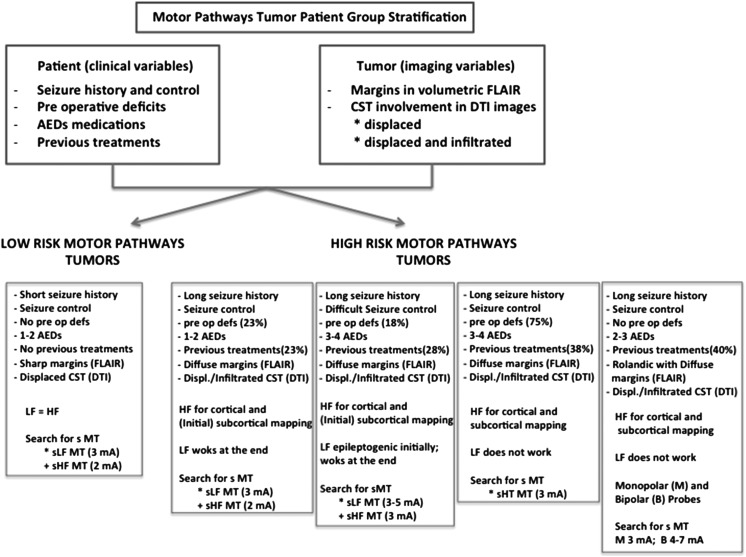
By looking at the mapping procedures adopted during surgery, the patients' related clinical and imaging features, and the clinical results, patients harboring a tumor involving the motor pathways can be clustered into 2 main groups (Fig. 6). The first group (low-risk motor tumors) comprises patients with a short history of seizures that are well controlled by one or a maximum of 2 AEDs, harbor tumors with well-demarcated (sharp) borders in FLAIR images, and have mostly displaced and minimally infiltrated CST in DTI images. In this setting, LF is efficient and, when delivered at threshold intensity (Group 1, subgroup A), allows better EOR (total resections: 84% in group1 vs 72% in Group 2; P = .05) and reduces the occurrence of intraoperative seizures (25% in Group 2: vs 16% in Group 1; P < .001). The second group (high-risk motor tumors) comprises patients with a long history of seizures who take high doses of several AEDs and is characterized by eventual poor seizure control, previous treatment, the appearance of diffuse and poorly demarcated tumor borders in FLAIR images, and highly displaced and infiltrated CST in DTI images. When LF is used in this high-risk group, it is usually not efficient and is associated with a high rate of permanent deficits (22.7% in Group 2 subgroups BI and BII) and a high occurrence of intraoperative seizures. The percentage of clinical seizures was indeed higher (50%) in Group 2 (subgroup B, only LF available and requiring high intensity) than in Group 1 (11% subgroup B) because HF was rapidly substituted when LF was found to be inefficient in this group. In this high-risk patient group, HF allows reliable mapping in all conditions with low permanent morbidity (2.7%) and low occurrence of intraoperative seizures (2%). In addition, subdividing the high-risk group into 4 subsets, according to their clinical and radiological features, allowed identification of each subgroup and determination of the best cortical and subcortical stimulation modalities and current intensities and the most appropriate probe (monopolar vs bipolar) choices to optimize mapping at the cortical and subcortical levels and reduce the occurrence of permanent deficits. Moreover, tailoring the use of LF and HF to clinical conditions has increased the percentage of high-risk patients who were taken to surgery, from 39.3% when only LF was available to 63% when both LF and HF were used (P < .001). Notably, the increased number of treated patients was associated with a significant reduction in permanent morbidity (from 22.7% in Group 2 to 2.7% in Group 1), a reduced incidence of intraoperative seizures (from 50% to 2%; P < .001), and a high rate of EOR (total resection >60%).
Fig. 6.
Motor pathways tumor patient group stratification. Patients with tumors involving motor pathways can be stratified into 2 main groups (low-risk and high-risk) according to clinical variables and tumor features defined by imaging. The most appropriate stimulation modality for each group is indicated. The high-risk group is further subdivided into 4 subgroups according to clinical and imaging parameters and the most appropriate neurophysiological strategy to be used intraoperatively. Abbreviations: LF, low-frequency stimulation (60 Hz); HF, high-frequency stimulation; sMT, subcortical motor threshold; sLF MT, subcortical motor threshold with LF; sHF MT, subcortical motor threshold with HF. The current intensity of sMT is indicated in milliamperes.
Neurophysiological Remarks: Intraoperative Use of LF and HF Stimulation Mapping
The use of LF and HF, as proposed here, requires technical nuances and careful interpretation by an in-house experienced neurophysiologist. The principal aspects are highlighted here (see Supplementary Materials for more details and Figs 7, 8, 9 in Supplementary Materials). High-sensitivity display of EMG allows detection of the earliest signs of muscle response to stimulation and thus prevents further increase of current intensity to generate evident movement of the body segment. This current limitation certainly helps reduce intraoperative seizures. In addition, multichannel EMG equipment can detect activity from multiple muscles, either induced by brain stimulation or by ictal activation, including less evident muscles such as the orofacial muscles. Detection of the earliest EMG signs of seizures, together with ECoG detection of AD, prompts interruption of stimulation and actions that usually succeed in aborting seizure activity.
At the cortical level, LF stimulation generates progressive motor unit recruitment with different features when compared with voluntary activation (Fig. 7B in Supplementary Materials). At the subcortical level, the time course of EMG activation, showed a progressive EMG recruitment only when stimulating CST fibers at the superficial (proximal to the cortex) loosing this feature when stimulating at deeper level (Fig. 7E in Supplementary Materials). HF stimulation requires EMG for visualization. HF stimulation generates MEPs, (ie, time-locked EMG responses to discrete stimulation) and shows similar morphology at any level of CST stimulation. Generally in HGGs, large tumors, previous surgeries/radiation, it is sometimes necessary to change polarity of stimulation to obtain responses at cortical level. For both LF and HF, the recruitment of cephalic (head-neck) and lower limb muscles generally requires higher current intensity (mean increment 2 mA) compared with upper limb muscles. Using an ultrasonic aspirator during LF stimulation at the subcortical level abolishes the responses19 but does not affect HF responses. At the subcortical level, assessment of the threshold current is crucial to estimating the minimal distance between the probe and the fibers and has to be adjusted according to clinical and radiological conditions (Fig. 6, high risk patients, 4 subgroups). In patients with well-demarcated tumors and history of well-controlled seizures, (Group 1, subgroup A), the LF threshold intensity (sLF-MT) corresponded to about 2 mA. In large tumors with edema and poorly controlled seizures, the sLF-MT increased about 2–4 mA (Group 1, subgroup B) and sometimes triggered seizures even before or without motor responses. With HF stimulation, the threshold intensity (sHF-MT, on average 2–3 mA) allowed mapping in all clinical contexts. When delivered by monopolar probe at the cortical level, HF generates responses from the primary motor and occasionally from somatosensory cortex, possibly due to a current spread. In these cases, the use of a bipolar probe evoked more focused responses (single or small groups of muscles) and no responses from the somatosensory cortex, but higher intensity is needed (range 4–7 mA). Subcortical stimulation with a monopolar probe at sHF-MT (3 mA) elicited multiple muscle responses even in distant body segments (eg, forearm and leg), suggesting a low spatial resolution of the stimulus. The bipolar probe, despite requiring higher intensity is more focal, allowing to extend the dissection closer to the CST (Fig. 5, 1Subgroup BIV).
The intensity of stimulation current must also be adjusted to the anesthesiological setting, being higher in asleep patients than in awake patients.
Discussion
This study reports the data obtained with the combined use of LF and HF stimulation for surgical removal of gliomas involving motor pathways. The analysis was performed on a large series of cases with a wide spectrum of clinical conditions. We adopted the strategy of applying the more effective stimulation protocol according to the conditions encountered at surgery. The successful neurophysiological protocols used at surgery were then related to the clinical context, defined by patient clinical history and tumor features, identified by tumor appearance on FLAIR conventional images and CST involvement on DTI images, and to clinical results, defined by occurrence of intraoperative seizures, EOR and percentage of permanent deficits. This allowed clustering patients with motor gliomas in clinical subgroups, defining for each the most effective neurophysiological protocol for guiding resection. Our data demonstrate that the integration of LF with HF stimulation tailored to the clinical context increases the number of patients who would benefit from surgery and significantly increases the EOR, while decreasing permanent morbidity and the occurrence of intraoperative seizures.
Historical Background
During surgery, it is crucial to distinguish the eloquent essential areas to be preserved from those that can be safely removed. The electrical stimulation of an eloquent area produces responses (positive site) consistent with its function (eg, muscular activation during motor cortex stimulation).4,20 Therefore, resection must preserve the positive sites.12 The challenge is finding the appropriate stimulating protocol to identify the different structures with different properties in the largest number of clinical conditions and reduce the bias of false-positive or false-negative sites. With regard to the motor system, the main issue is preserving the corticospinal system. Two stimulation paradigms evolved for intraoperative guidance: the 60 Hz technique (low frequency) and the “train-of-five” technique. At present, LF stimulation, introduced by Penfield, is widely used. The To5, which delivers short trains of pulses at high frequency, was originally conceived as a constant monitor for functional integrity of the descending motor pathways13 rather than a tool for mapping. Most surgical teams rely on either of these 2 techniques. In addition, there is a very strict association between the stimulating protocol and the probe (LF: bipolar probe; HF: monopolar probe),12,20–28 and few data are available on the comparison between HF and LF.7,8,29 No data are available on integrating the choice of stimulation modality with the clinical context. Gliomas are, in fact, highly heterogeneous tumors, and their degree of heterogeneity increases as a result of the treatments the patients receive. With regard to tumors within the motor pathways, the degree of involvement of the corticospinal tract is generally quite variable, determining the various spectra of patients’ clinical history and presentation and impacting the level of excitability of the motor pathways at surgery. Clinical features, such as seizure history, number and doses of AEDs, seizure control, and previous treatments, are the clinical parameters that offer indication for the level of CST excitability that may be found intraoperatively. In addition, preoperative MR studies, such as the appearance of tumor on volumetric FLAIR images or DTI reconstruction, may provide the surgeon with a view of CST involvement and represents a useful tool for planning intraoperative surgical and neurophysiological strategy,9
This study compared HF with LF by investigating their clinical field of application, the advantages, the disadvantages, and the overlapping features. The probe was analyzed independently of the stimulating protocol to determine the best probe-frequency match for different clinical conditions (patient clinical history and tumor appearance on imaging) and evaluated in terms of clinical outcome (percentage of intraoperative seizures, rate of permanent morbidity, EOR) and the number of patients who underwent successful surgery.
The analysis of the data suggests 2 levels of discussion: a methodological level, in which the technical aspect of the stimulation is discussed, and a clinical level, in which the advantages in terms of clinical feasibility and outcome are discussed.
Analysis of the Frequency of Stimulation and Probes
Subdividing the 2 groups into different subsets allowed assessment of the advantages and disadvantages of HF and LF for the different clinical conditions related to patient clinical history and tumor. Both the frequency of stimulation and the probe emerged as crucial parameters for the mapping procedure. In tumors with sharp borders on FLAIR images, no or little CST infiltration in DTI images, and stable cortical excitability, as suggested by short seizure history, optimal seizure control by one or few AEDs, LF stimulation is maximally efficient (Group 1/Subgroup A; Group 2/Subgroup A), and optimized by using the threshold current (total EOR Group 1> Group 2) Group 1/subgroup B shows 4 different subsets corresponding to the different clinical settings (history of several seizures, edema, infiltrative behavior of the tumor at imaging, and altered cortical excitability) in which LF stimulation failed at mapping the cortical or subcortical level, while HF was conversely very efficient. In tumors with diffuse margins and high CST infiltration, HF allowed resection after considerable decompression until LF resumed its efficacy. A question arises on whether the efficiency of HF was optimized by using the monopolar probe, which presumably stimulated a larger area than the bipolar probe and reached fibers with tumor infiltration and edema that were far from the electrode. In fact, the same fibers became responsive to LF after decompression. LF stimulation was efficient but ictogenic in patients with altered excitability (Group 1/subgroup BII, long seizure history and poor seizure control), while HF stimulation was optimal; this may have been due to the frequency. Short trains at high frequency (250–500 Hz) are very effective for exciting the motor structures, while LF (60 Hz) needs long trains (1–4 s), at high risk of seizures over a tissue with aberrant excitability. In the last 2 subsets (Group 1/subgroup BIII-IV) of high-risk patients (highly diffuse tumor, edema, highly infiltrated CST, seizures, motor deficits), LF completely failed mapping, while HF was efficient. Finally, the last subset (Group 1/subgroup BIV) highlighted the advantages of delivering HF with a more focal stimulus (bipolar probe). Overall, these data suggest that HF stimulation is an efficient tool for testing the motor pathways in all clinical settings, with selection of the cortical and subcortical MT and probe (monopolar vs bipolar) being based on clinical conditions. The use of LF should be restricted to patients with a short history of seizures and tumor with limited CST infiltration. In these cases, LF has the advantage of delivering the stimulus focally with a bipolar probe.
Clinical Impact
The clinical impact of combining HF and LF stimulation needs to be evaluated for feasibility of mapping (number of cases in which reliable mapping is obtained) and clinical outcome (EOR and rate of permanent morbidity).
Generally, resection is continued until positive sites are encountered. The concept that resection can be safely performed in areas not responsive to electrical stimulation (negative foci) assumed that the absence of response accounted for the absence of a neurological function.4 However, the occurrence of permanent post-operative deficits, leads to consider the possible inadequacy of the stimulating procedure has yielded to the occurrence of “false negative foci”. In our experience, in fact, where LF stimulation failed, thus reporting a false negative site (in high risk patients, 63% of Group 1), HF stimulation was conversely efficient. In 89% of these patients (Group 1-BI and Group 1-BII) LF stimulation resumed after decompression of the motor pathways. Had the negative-site approach been used in these cases, permanent deficits could have occurred. The presumable occurrence of LF false-negative sites at the beginning of surgery should be suspected in cases with altered CST excitability and may be predicted by looking at both clinical parameters (eg, long history of seizures, poor seizure control, large number of AEDs, or previous treatment) and tumor features (eg, diffuse infiltration on FLAIR images and high CST involvement on DTI images). In these conditions, which defined the high-risk motor group patients, the use of LF is not the first choice because of its association with high permanent morbidity (22.7%) and high occurrence of intraoperative seizures (50%). In this setting, HF should be considered the first choice because it is efficient in all conditions, with low permanent morbidity (2.7%) and low incidence of intraoperative seizures (2%). As stated in the previous paragraph, in this setting, the efficiency of HF may be due either to the use of the monopolar probe, presumably stimulating a larger area with respect to the bipolar probe, reaching fibers suffering by tumor infiltration and edema and distant to the electrode (subgroup BI-I); alternatively, in tumors with altered cortical exitability (subgroup BI-II) may be due to due to its frequency (short trains), which is highly efficient in exciting motor structures and is less ictogenic. Notably, with the combined use of LF and HF, the percentage of high risk patients who were successfully mapped and benefit from surgery increased from 39% of the historical group (only LF available) to 63% (both HF and LF available).
This study clearly demonstrates that the search for the threshold current, suggesting the minimal distance from the essential fibers,30–35 is mandatory for increasing EOR and decreasing permanent morbidity. Total resection was in fact accomplished with LF in 72% of sharp tumors (Group 2/subgroup A) and increased to 84.1% when LF-threshold current was used (Group 1/subgroup A). The use of HF and LF stimulation at threshold current intensity increased the rate of total resection in infiltrative tumors (from 60 to 70%), decreasing permanent deficits (from 9% Group 2 Subgroup B, to 2.1% Group 1).
Procedures guided by subcortical mapping are associated with occurrence of transient deficits (from 10.4% to 49.6% in different studies).1,12,31,36–38 The finding of positive subcortical sites is generally associated with a higher rate of permanent deficits (from 7.4% to 15.1% according to various series). Our data show that it is possible to further decrease this percentage to 1.8%, even in high risk cases, by using stimulation either alternated with resection or coupled with it and using threshold current with the appropriate probe.
Clinical Constraints
The combined use of LF and HF protocols adopted in the present series showed the advantage of tailoring the stimulation protocol to clinical conditions, enhancing motor pathways identification at both cortical and subcortical levels, and targeting resection to the MT to further extend the margin of resection. The use of MT EMG responses, by decreasing the current intensity needed to evoke a response, significantly reduces the incidence of intraoperative seizures. However, there are 2 limitations: the need for complex equipment provided with wide EMG recording to detect evoked responses during surgery and the need for an inhouse, experienced neurophysiologist to carefully interpret the responses. EMG recording is necessary for distinguishing induced movement from voluntary movement, to monitor the superficial or deep level of stimulation, to record MEPs induced by HF simultaneously in different muscles, and to recognize different muscle recruitment when changing probe.
The intensity of current should be adjusted to the anesthesia setting (higher in asleep conditions) and to the muscular district (cephalic, upper or lower limb) to be tested. Using the appropriate frequency and probe is suggested when mapping nonprimary motor or sensory areas to avoid current spread and to relate responses to the correct area.
Conclusions
Our data suggest that the combined use of LF-HF stimulation, when tailored to the clinical conditions identified by patient clinical history and tumor features as shown by imaging, increases the feasibility of mapping and the number of patients who would be candidates for surgery. The frequency of stimulation and the choice of probe must be considered independently. The choice of the appropriate stimulation and probe depends upon the clinical context and may change within the same surgery according to ongoing modifications during the procedure. In tumors involving motor pathways, it seems reasonable to suggest HF as the best frequency paradigm to stimulate the motor system.39 In conclusion, being the chance to identify the “positive” sites strictly related to the stimulation paradigm, it is mandatory that both the surgeon and the clinical neurophysiologist know the advantages and limitations of each stimulation modality and probe for various clinical conditions.
Supplementary Material
Funding
The work was supported by grants from the Italian Ministry of Health, Ricerca Finalizzata RF-2009-1530888 (to AF and LB), 2010 (to LB, GC, AF); Associazione Italiana Ricerca sul Cancro (to LB); Fondazione Berlucchi (to LB); Fondazione Italo Monzino (to LB).
Conflict of interest statement. None declared.
Supplementary Material
References
- 1.Smith JS, Chang EF, Lamborn KP, et al. Role of extent of resection in the longterm outcome of low grade hemispheric gliomas. J Clin Oncol. 2008;10:1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 2.Bello L, Fava E, Carrabba G, Papagno C, Gaini SM. Present day's standards in microsurgery of low-grade gliomas. Adv Tech Stand Neurosurg. 2010;35:113–157. doi: 10.1007/978-3-211-99481-8_5. [DOI] [PubMed] [Google Scholar]
- 3.Duffau H. Introduction. Surgery of gliomas in eloquent areas: from brain hodotopy and plasticity to functional neurooncology. Neurosurg Focus. 2010;28(2) doi: 10.3171/2009.12.focus.feb2010.intro. Intro. [DOI] [PubMed] [Google Scholar]
- 4.Sanai N, Berger MS. Intraoperative stimulation techniques for functional pathway preservation and glioma resection. Neurosurg Focus. 2010;28(2):E1. doi: 10.3171/2009.12.FOCUS09266. [DOI] [PubMed] [Google Scholar]
- 5.Bello L, Gambini A, Castellano A, et al. Motor and language DTI Fiber Tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage. 2008;39(1):369–382. doi: 10.1016/j.neuroimage.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 6.Robles SG, Gatignol P, Lehèricy S, Duffau H. Long-term brain plasticity allowing a multistage surgical approach to World Health Organization Grade II gliomas in eloquent areas. J Neurosurg. 2008;109(4):615–624. doi: 10.3171/JNS/2008/109/10/0615. [DOI] [PubMed] [Google Scholar]
- 7.Szelenyi A, Senft C, Jardan M, et al. Intra-operative subcortical electrical stimulation: a comparison of two methods. Clin Neurophysiol. 2011;122(7):1470–1475. doi: 10.1016/j.clinph.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 8.Kombos T, Suess O, Kern BC, et al. Comparison between monopolar and bipolar electrical stimulation of the motor cortex. Acta Neurochir (Wien) 1999;141(12):1295–1301. doi: 10.1007/s007010050433. [DOI] [PubMed] [Google Scholar]
- 9.Castellano A, Bello L, Michelozzi C, et al. Role of diffusion tensor magnetic resonance tractography in predicting the extent of resection in glioma surgery. NeuroOncol. 2012;14(2):192–202. doi: 10.1093/neuonc/nor188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bello L, Gallucci M, Fava M, et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery. 2007;60(1):67–80. doi: 10.1227/01.NEU.0000249206.58601.DE. comments: 80–82. [DOI] [PubMed] [Google Scholar]
- 11.Duffau H, Capelle L, Sichez N, et al. Intraoperative mapping of subcortical language pathways using direct stimulation: an anatomo-functional study. Brain. 2002;125:199–214. doi: 10.1093/brain/awf016. [DOI] [PubMed] [Google Scholar]
- 12.Keles GE, Lundin DA, Lamborn KR, Chang EF, Ojemann G, Berger MS. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004;100(3):369–375. doi: 10.3171/jns.2004.100.3.0369. [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi M, Nadstawek J, Langenbach U, Bremer F, Schramm J. Effects of four intravenous anesthetic agents on motor evoked potentials elicited by magnetic transcranial stimulation. Neurosurgery. 1993;33(3):407–415. doi: 10.1007/978-3-642-78801-7_46. [DOI] [PubMed] [Google Scholar]
- 14.Szelenyi A, Hattingen E, Weidauer S, Seifert V, Ziemann U. Intraoperative motor evoked potential alteration in intracranial tumor surgery and its relation to signal alteration in postoperative magnetic resonance imaging. Neurosurgery. 2010;67(2):302–313. doi: 10.1227/01.NEU.0000371973.46234.46. [DOI] [PubMed] [Google Scholar]
- 15.Proposal for Revised Clinical and Electroencephalographic Classification of Epileptic Seizures. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 16.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Mori S, Frederiksen K, van Zijl PC, Stieltjes B, Kraut MA, Solaiyappan M. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Ann Neurol. 2002;51(3):377–380. doi: 10.1002/ana.10137. [DOI] [PubMed] [Google Scholar]
- 18.Xue R, van Zijl PC, Crain BJ, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magn Reson Med. 1999;42(6):1123–1127. doi: 10.1002/(sici)1522-2594(199912)42:6<1123::aid-mrm17>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Carrabba G, Mandonnet E, Fava E, et al. Transient inhibition of motor function induced by the Cavitron ultrasonic surgical aspirator during brain mapping. Neurosurgery. 2008;63(1):E178–E179. doi: 10.1227/01.NEU.0000335087.85470.18. comments: E179. [DOI] [PubMed] [Google Scholar]
- 20.Szelenyi A, Bello L, Duffau H, et al. Intraoperative electrical stimulation in awake craniotomy: methodological aspects of current practice. Neurosurg Focus. 2010;28(2):E7. doi: 10.3171/2009.12.FOCUS09237. [DOI] [PubMed] [Google Scholar]
- 21.Chacko AG, Thomas SG, Babu KS, et al. Awake craniotomy and electrophysiological mapping for eloquent area tumours. Clin Neurol Neurosurg. 2013;115(3):329–334. doi: 10.1016/j.clineuro.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Krieg SM, Schnurbus L, Shiban E, et al. Surgery of highly eloquent gliomas primarily assessed as non-resectable: risks and benefits in a cohort study. BMC Cancer. 2013;2:13–51. doi: 10.1186/1471-2407-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prabhu SS, Gasco J, Tummala S, Weinberg JS, Rao G. Intraoperative magnetic resonance imaging-guided tractography with integrated monopolar subcortical functional mapping for resection of brain tumors. Clinical article. J Neurosurg. 2011;114(3):719–726. doi: 10.3171/2010.9.JNS10481. [DOI] [PubMed] [Google Scholar]
- 24.Suess O, Suess S, Brock M, Kombos T. Intraoperative electrocortical stimulation of Brodman area 4: a 10-year analysis of 255 cases. Head Face Med. 2006;3:2–20. doi: 10.1186/1746-160X-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schucht P, Moritz-Gasser S, Herbet G, Raabe A, Duffau H. Subcortical electrostimulation to identify network subserving motor control. Hum Brain Mapp. 2013;34(11):3023–3030. doi: 10.1002/hbm.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talacchi A, Turazzi S, Locatelli F, et al. Surgical treatment of high-grade gliomas in motor areas. The impact of different supportive technologies: a 171-patient series. J Neurooncol. 2010;100(3):417–426. doi: 10.1007/s11060-010-0193-x. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi F, Takahashi H, Teramoto A. Intra-operative detection of motor pathways using a simple electrode provides safe brain tumor surgery. J Clin Neurosci. 2007;14(11):1106–1110. doi: 10.1016/j.jocn.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi F, Takahashi H, Teramoto A. Navigation-assisted subcortical mapping: intraoperative motor tract detection by bipolar needle electrode in combination with neuronavigation system. J Neurooncol. 2009;93(1):121–125. doi: 10.1007/s11060-009-9847-y. [DOI] [PubMed] [Google Scholar]
- 29.Kombos T, Suess O, Funk T, Kern BC, Brock M. Intra-operative mapping of the motor cortex during surgery in and around the motor cortex. Acta Neurochir (Wien) 2000;142(3):263–268. doi: 10.1007/s007010050034. [DOI] [PubMed] [Google Scholar]
- 30.Bello L, Fava E, Casaceli G, et al. Intraoperative mapping for tumor resection. Neuroimaging Clin N Am. 2009;19(4):597–614. doi: 10.1016/j.nic.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Bello L, Castellano A, Fava E, et al. Intraoperative use of diffusion tensor imaging fibertractography and subcortical mapping for resection of gliomas: technical considerations. Neurosurg Focus. 2010;28(2):E6. doi: 10.3171/2009.12.FOCUS09240. [DOI] [PubMed] [Google Scholar]
- 32.Bertani G, Fava E, Casaceli G, et al. Intraoperative mapping and monitoring of brain functions for the resection of low-grade gliomas: technical considerations. Neurosurg Focus. 2009;27(4):E4. doi: 10.3171/2009.8.FOCUS09137. [DOI] [PubMed] [Google Scholar]
- 33.Nossek E, Korn A, Shahar T, et al. Intraoperative mapping and monitoring of the corticospinal tracts with neurophysiological assessment and 3-dimensional ultrasonography-based navigation. Clinical article. J Neurosurg. 2011;114(3):738–746. doi: 10.3171/2010.8.JNS10639. [DOI] [PubMed] [Google Scholar]
- 34.Ohue S, Kohno S, Inoue A, et al. Accuracy of diffusion tensor magnetic resonance imaging-based tractography for surgery of gliomas near the pyramidal tract: a significant correlation between subcortical electrical stimulation and postoperative tractography. Neurosurgery. 2012;70(2):283–293. doi: 10.1227/NEU.0b013e31823020e6. [DOI] [PubMed] [Google Scholar]
- 35.Seidel K, Beck J, Stieglitz L, Schucht P, Raabe A. The warning-sign hierarchy between quantitative subcortical motor mapping and continuous motor evoked potential monitoring during resection of supratentorial brain tumors. J Neurosurg. 2013;118(2):287–296. doi: 10.3171/2012.10.JNS12895. [DOI] [PubMed] [Google Scholar]
- 36.Carrabba G, Fava E, Giussani C, et al. Cortical and subcortical motor mapping in rolandic and perirolandic glioma surgery: impact on postoperative morbidity and extent of resection. J Neurosurg Sci. 2007;51(2):45–51. [PubMed] [Google Scholar]
- 37.Talacchi A, Turazzi S, Locatelli F, et al. Surgical treatment of high-grade gliomas in motor areas. The impact of different supportive technologies: a 171-patient series. J Neurooncol. 2010;100(3):417–426. doi: 10.1007/s11060-010-0193-x. [DOI] [PubMed] [Google Scholar]
- 38.Wilden JA, Voorhies J, Mosier KM, O'Neill DP, Cohen-Gadol AA. Strategies to maximize resection of complex, or high surgical risk, low-grade gliomas. Neurosurg Focus. 2013;34(2):E5. doi: 10.3171/2012.12.FOCUS12338. [DOI] [PubMed] [Google Scholar]
- 39.Deletis V, Sala F. Subcortical stimulation (mapping) of the corticospinal tract. Clin Neurophysiol. 2011;122(7):1275–1276. doi: 10.1016/j.clinph.2011.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.