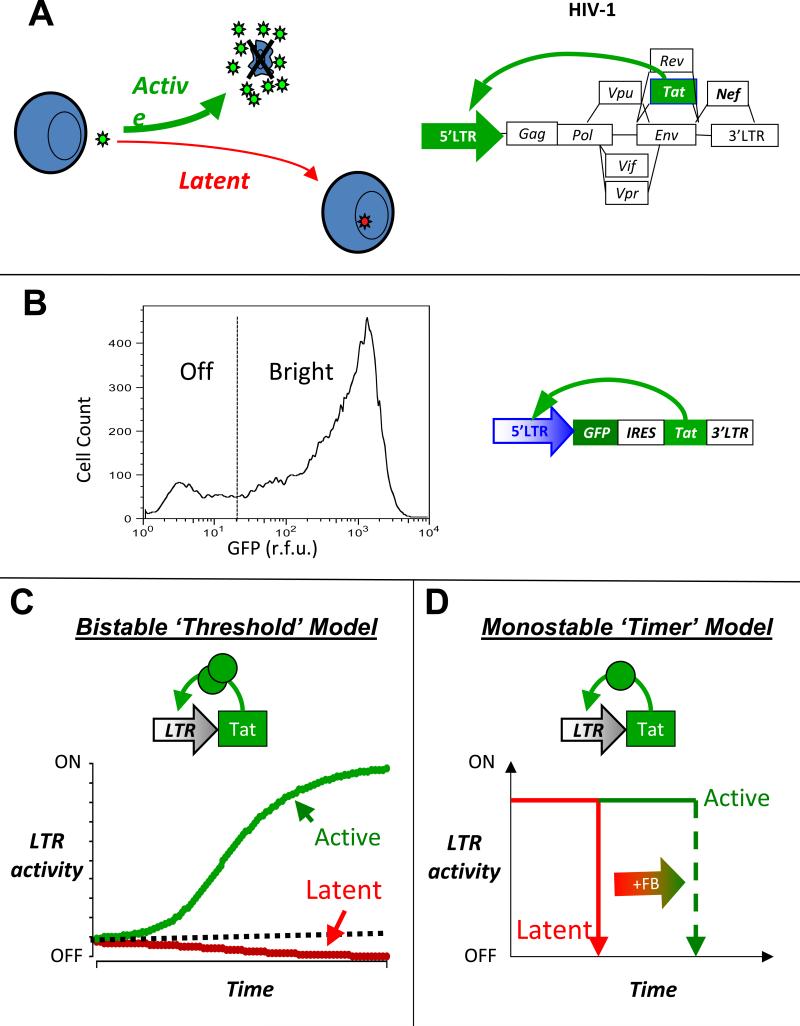
Figure 1. The HIV-1 provirallatency decision oand two potential decision-making mechanisms.
(A) Schematic of HIV-1 infecting a CD4+ T cell and ‘choosing’ between active replication and proviral latency (left) and schematic of the HIV-1 proviral genome where the HIV-1 Tat positive feedback is essential for active replication and entry into proviral latency (right). (B) A minimal Tat circuit LTR-GFP-IRES-Tat (LGIT) can generate a rcation in GFP between two states: Off and Bhght(12). Flow cytometry histogram of a at cell clonal population expressing LGIT from a single locus and exhibiting a developmental bifurcation; despite all cells having the same integration of LGIT, one subpopulation of cells does not express GFP and Tat while another subpopulation of cells does express GFP and Tat. The bifurcation is not consistent with chromatin silencing or position effect variegation but is consistent with stochastic fluctuations in Tat (12). (C) The bistability model for developmental bifurcation. If a transcriptional positive-feedback circuit encodes a self-cooperative threshold (e.g. homodimerizationof the transactivator or multiple DNA binding sites that must all be bound by the transactivator), the circuit can exhibit bistability (the ability to stably rest in two alternate states). If the transactivator levels are above the threshold (dashed line), the circuit self-perpetually transcribes increasing amounts of transactivator and remains stably in an active state (green line). If transactivator levels are below the threshold, feedback regulation cannot be completed and any available transactivator decays away at its intrinsic half-life (red line). (D) An alternate model of kinetic partitioning (or a ‘timer’ switch) for developmental bifurcation. In this model, the positive-feedback loop is not required to encode a self-cooperativity threshold and all trajectories eventually fall to an off state (red) and the strength of positive feedback determines the duration of time that a circuit resides in the on state (green).