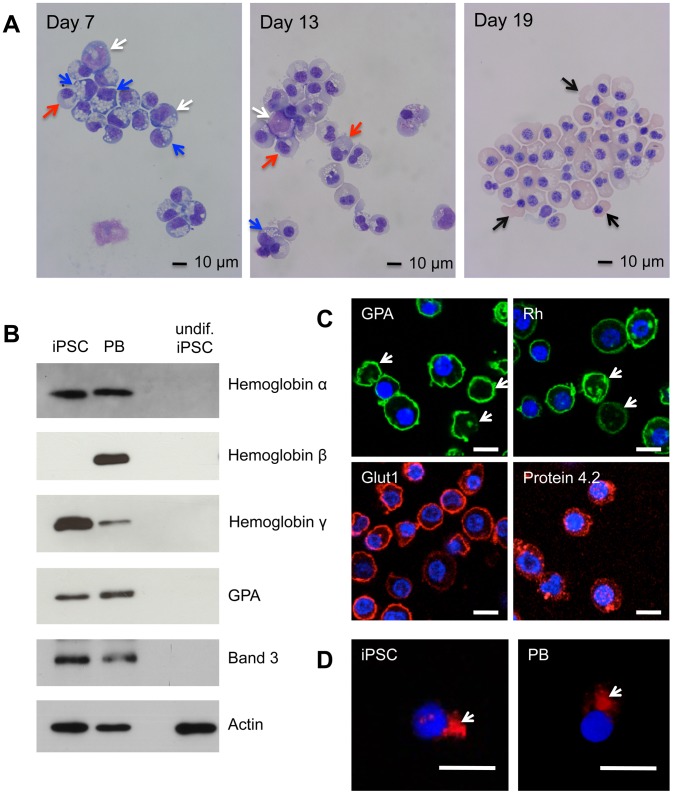
Figure 1. Erythroid differentiation of C19 iPSC CD34+ cells.
C19 and adult peripheral blood [PB] CD34+ cells were incubated for up to 21 days in our three-stage erythroid culture system. (A) Morphological analysis of cells stained with May-Grundwal Giemsa reagent on day 7, 13 and 19 in culture. Arrows, white proerythroblasts, blue basophillic erythroblasts, red polychromatic erythroblasts, black orthochromatic erythroblasts. (B) Western blot of iPSC and PB erythroid cells at day 19 in culture, and undifferentiated [undif] iPSCs, probed with antibodies to α-,β- and γ-globin, GPA and Band 3. Antibodies to actin were used as a protein loading control. Numbers on left are size markers. (C) iPSC erythroid cells at day 19 in culture probed with antibodies to GPA, Rh, GLUT1 and Protein 4.2, followed by compatible secondary antibodies with Alexa Fluor 488 (green) or Alexa Fluor 635 phalloidin (red). Arrows indicate reticulocytes. (D) Erythroid cells differentiated from C19 iPSC and PB progenitors were incubated with Alexa Fluor 635 phalloidin conjugated actin antibody (red). Arrows indicate contractile actin rings. Nuclear DNA was stained with blue-fluorescent DAPI. Images were obtained using a Leica SP5 confocal microscope. Scale bars 10 µm.