Figure 1.
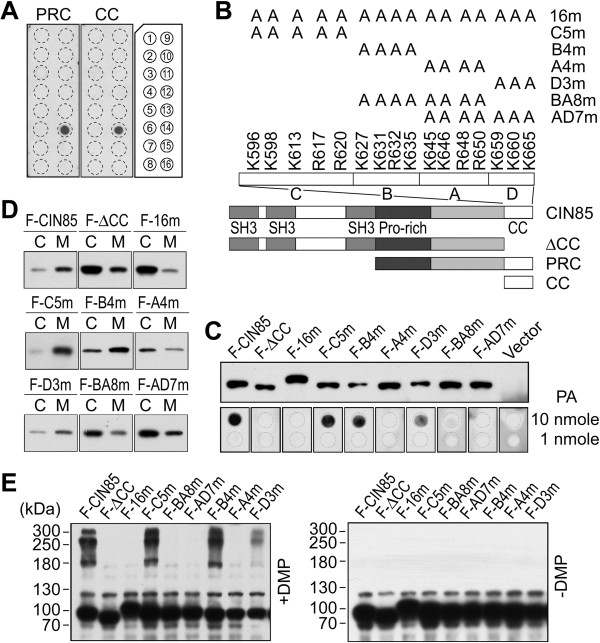
K645, K646, R648 and R650 in coiled-coil domain are essential for CIN85-phosphatidic acid interaction and membrane association. (A) Interaction of the coiled-coil domain with phosphatidic acid. The coiled-coil domain (CC) and the C-terminal fragment (PRC) were eGFP-tagged, expressed and purified from HEK293 cells to blot PIP StripTM membrane. The phospholipids on the PIP StripTM membrane are: 1, lysophosphatidic acid; 2, lysophosphatidylcholine; 3, phosphatidylinositol; 4, PtdIns(3)P; 5, PtdIns(4)P; 6, PtdIns(5)P; 7, phosphatidylethanolamine; 8, phosphatidylcholine; 9, sphingosine 1-phosphate; 10, PtdIns(3,4)P; 11, PtdIns(3,5)P; 12, PtdIns(4,5)P; 13, PtdIns(3,4,5)P; 14, phosphatidic acid; 15, phosphatidylserine; 16, blank. (B) The point mutations of the basic amino acids in the coiled-coil domain and other truncation mutations. The 16 basic amino acids in coiled-coil domain were divided into 4 groups (C5, B4, A4 and D3) and were mutated to alanine (A). (C) Interaction of CIN85 mutants with phosphatidic acid. Phosphatidic acid (PA) was dotted on nitrocellulose membrane at 1 and 10 nmole. The Western blot showed the relative protein level of each mutant used to blot the phosphatidic acid membrane. The mutants were Flag-tagged. (D) The association of CIN85 mutants with intracellular membrane vesicles. M, the membrane fraction of ultracentrifugation; C, the cytosolic fraction of ultracentrifugation. (E) Chemical cross-link of CIN85 mutants by dimethylpimelimidate (DMP). +DMP, cell lysate was treated with DMP; −DMP, control cell lysate. The numbers indicate the molecular weight markers.
