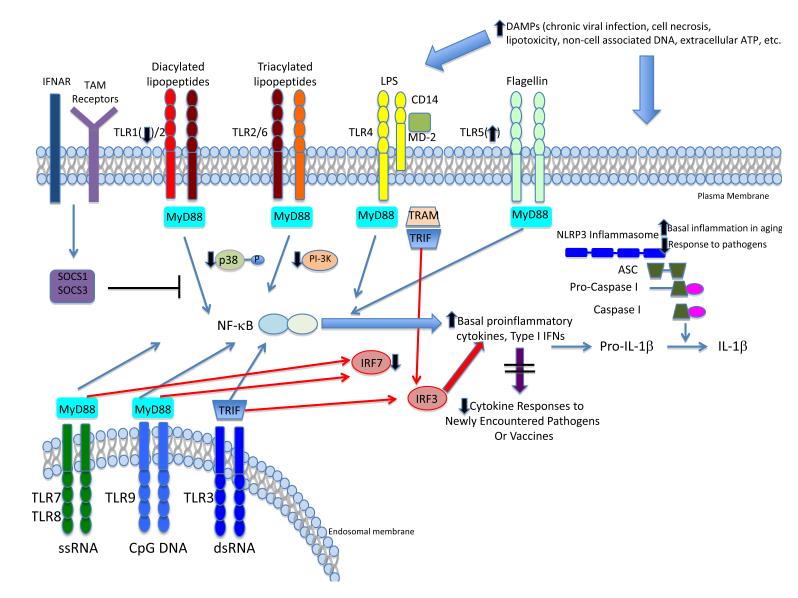
Figure 2. Effects of ageing on innate immune PRR signalling.
TLR and NLRP3 signalling pathways are depicted, with NF-κB-dependent pathways indicated in blue and type I interferon pathways indicated in red. With aging, elevated levels of PRR ligands (DAMPs) arising from cell damage, necrosis, lipotoxicity or other causes contribute to an elevated pro-inflammatory state, as manifested by increased basal levels of proinflammatory cytokines that may result in part from both TLR and NLRP3 signalling. Such basal activation may restrict responsiveness to new pathogens or vaccines, resulting in innate immune failure and impaired adaptive immune responses. Changes in protein expression for TLR1 and TLR5 in humans are indicated; results in mice suggest either a widespread decrease in TLR gene expression, or preserved expression with changes in intracellular signaling proteins (e.g. p38 MAP kinase). Abbreviations: TLR—Toll-like Receptor; IFNAR—type I Interferon receptor; MyD88—myeloid differentiation primary response 88; TRIF—Toll/IL1 receptor domain-containing adapter-inducing interferon-β; TRAM—TRIF-related adaptor molecule; IRF3—interferon regulatory factor 3; IRF7—interferon regulatory factor 7; NLRP3—NOD-like receptor family, pyrin domain containing 3; ASC—Apoptosis-associated speck-like protein containing a CARD.