Figure 12.
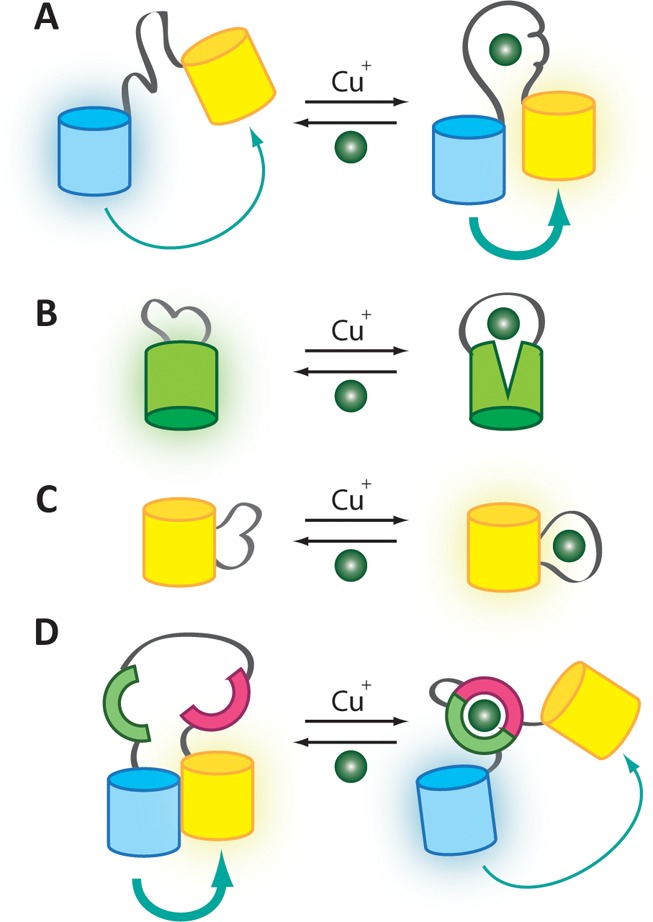
Mechanism of metal ion sensing by genetically encoded probes for Cu+. (A) AMT1-FRET, Ace1-FRET, and Mac1-FRET have a cysteine-rich Cu+-binding domain between a CFP/YFP FRET pair such that metal binding results in an increased FRET signal. (B) Cu+ binding to EGFP-Amt1 distorts the β-barrel of EGFP and decreases fluorescence. (C) YFP-Ace1 and related sensors have the Cu+-binding domain of Ace1 inserted between two strands of EYFP. Cu+ binding alters the local environment of the chromophore and leads to an increased fluorescent signal. (D) The eCALWY Zn2+ sensor platform can be tuned for improved selectivity toward Cu+. In the absence of Cu+, association between two FPs produces a FRET signal. CU+-induced association between the metal binding domains of Atox1 and WD4 changes the structure of the sensor and results in a decreased FRET signal.
