Figure 9.
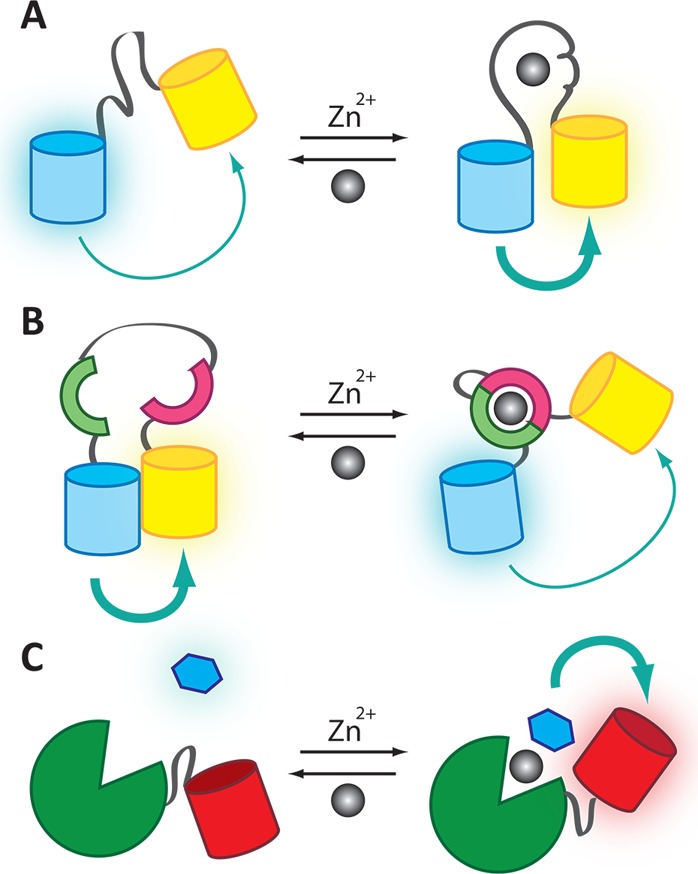
Mechanisms of metal ion sensing by genetically encoded and hybrid probes for Zn2+. (A) The Zap and Zif families consist of one or two Zn2+-finger domains between two FPs. Zn2+ binding induces a conformational change in the Zn2+-finger that leads to a change in FRET ratio. (B) The eCALWY family uses Zn2+ binding domains from Atox1 and WD4. The two FPs associate in the absence of Zn2+, but Zn2+ binding causes association of the binding domains and reduces the FRET efficiency. (C) The hybrid probe CA-FP has an FP linked to CA. When Zn2+ binds to CA, an exogenously added dapoxyl sulfonamide (blue hexagon) can bind to an open site on the Zn2+ ion, leading to a FRET response between the small-molecule fluorophore and the FP.
