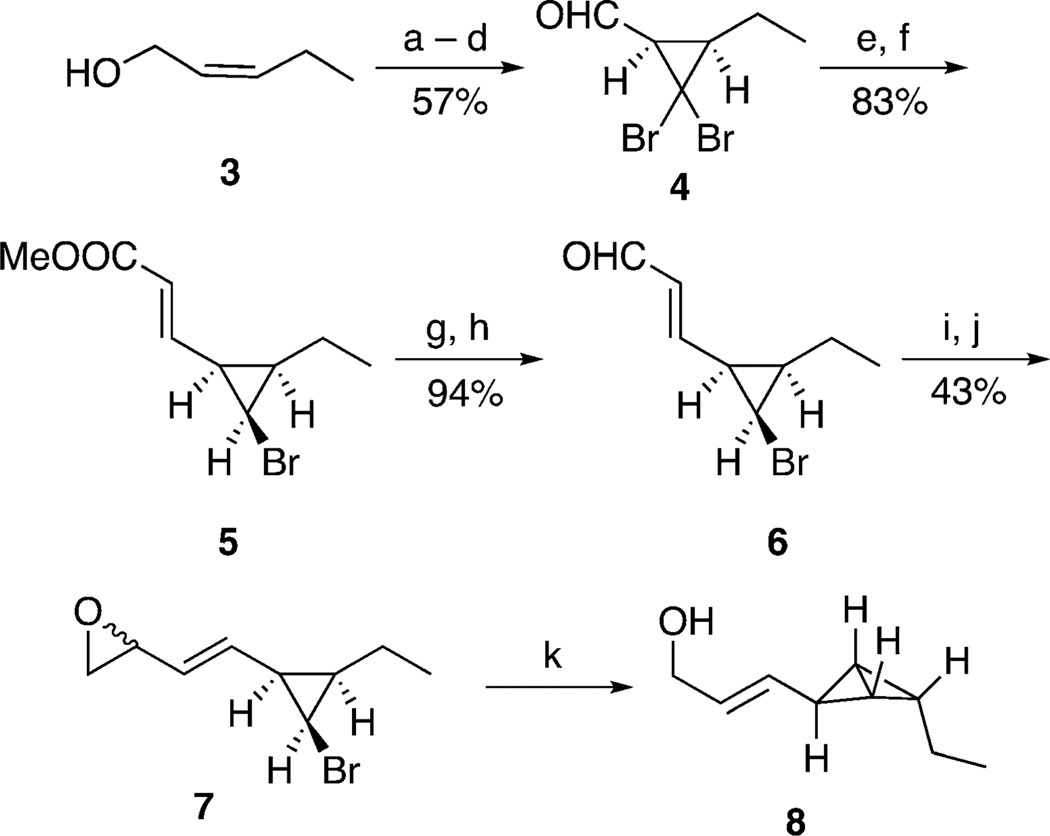
Scheme 3.
Synthesis of bicyclobutane 8. a) TBSCl, Et3N, CH2Cl2, RT, 94%; b) CHBr3, BnEt3NCl, 50% NaOH (aq), RT, 80%; c) TBAF, THF, RT, 95%; d) SO3·Pyr, DMSO, iPr2EtN, CH2Cl2, −20°C, 79%; e) (EtO)2P(O)CH2CO2CH3, NaH, THF −78 °C, 87%; f) Et3B, Ph3SnH, PhMe, −78 °C, 95%; g) DIBAl-H, CH2Cl2, −78°C, 95%; h) MnO2, CH2Cl2, RT, 99%; i) ICH2Cl, nBuLi, THF, −78°C, 51%; j) NaH, THF, −78 °C, 84%; k) i. nBuLi, THF, −78 °C, ii. [CuI·2 (LiCl)], THF, −78 to −20 °C. DIBAl-H=diisobutylaluminium hydride, DMSO=dimethylsulfoxide, Pyr=pyridine, TBAF=tetra-n-butylammonium fluoride, TBS=tert-butyldimethylsilyl, THF=tetrahydrofuran.