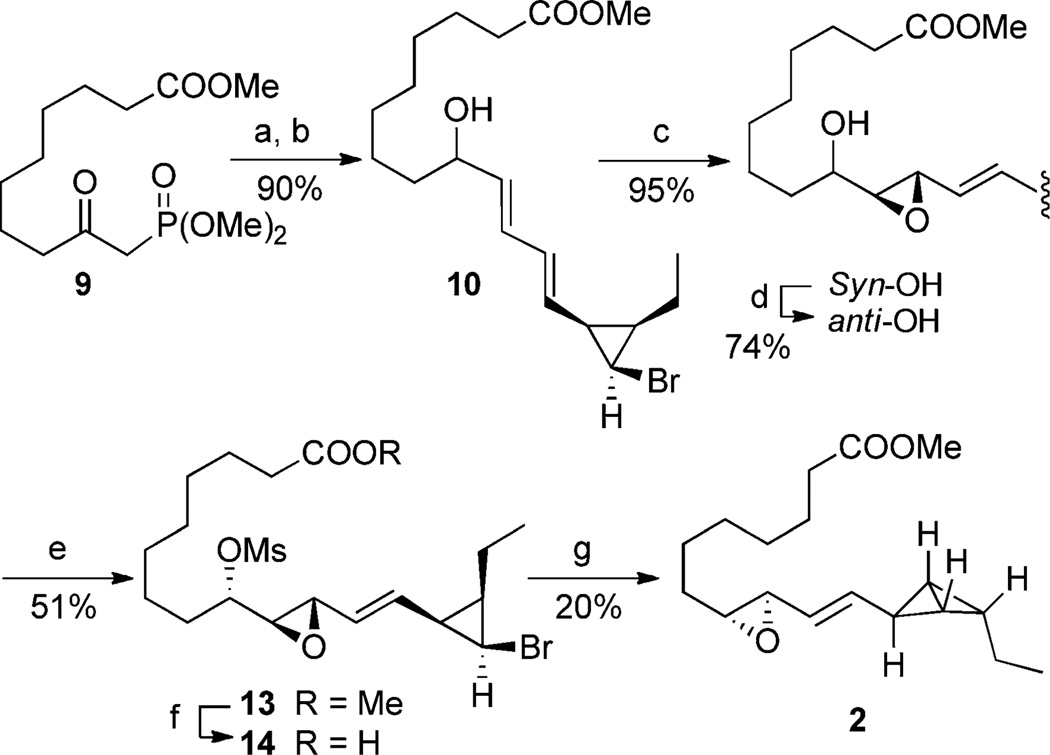
Scheme 4.
Synthesis of bicyclobutane 2. a) KHMDS, THF, −78°C→RT, then 6, 92%; b) NaBH4, CeCl3, MeOH, RT, 98%; c) DMDO, CH2Cl2, 0°C, 95%; d) PPh3, DIAD, p-nitrobenzoic acid, RT then K2CO3, MeOH, 74%; e) MsCl, Et3N, DMAP, CH2Cl2, 0°C to RT, 56%; f) LiOH (aq), THF, 60°C; g) tBuLi, THF, −78 to −20°C, then CH2N2, 20%. DIAD=diisopropyl azodicarboxylate, DMAP=4-dimethylaminopyridine, DMDO=dimethyl dioxirane, HMDS=hexamethyldisilazide, Ms=methanesulfonyl.