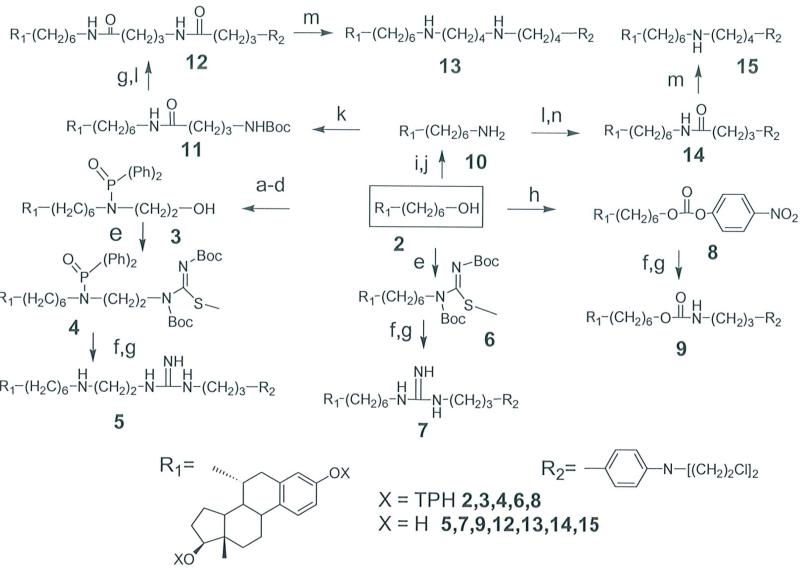
Scheme 1.
Synthesis of estradiol linked aniline mustards. The starting compound 2 is shown in the box (center). Reagents and conditions: (a) methanesulfonyl chloride, DIEA, THF; (b) LiBr, TEA, DMF, 60°C; (c) Ph2P(O)NHCH2CH20TBDMS, NaH, TBAB, Ph-H, 60°C; (d) TBAF, THF; (e) Boc-NHC(=N-Boc)SCH3, PPh3, DIP AD, THF; (f) 4-(N,N-bis-2-chloroethylamino-phenyl)-propylamine, THF/H20(90: 10), reflux; (g) HCl/dioxane, CH2Cl2; (h) p-nitrophenylchloroformate, DIEA, THF; (i) phthalimide, DIP AD, PPh3, THF; (j) Hydrazine, EtOH, reflux; (k) 4-(N-Boc)n-butyl(N-hydroxysuccinimide)ester, TEA, DMF; (1) 4-(N,N-bis-chloroethylamino-phenyl)butyl(N -hydroxysuccinimide )ester, TEA, DMF; (m) BH3S(CH3)2 THF, HCl; (n) HCl, THF. DIEA= Diisopropylethyl amine; TEA= Triethyl amine; TBAB= Tetrabutylammonium bromide;TBAF= Tetrabutylammonium fluoride; DIP AD= Diisopropylethyl azadicarboxylate; DMF= Dimethylformamide; THF= Tetrahydrofuran