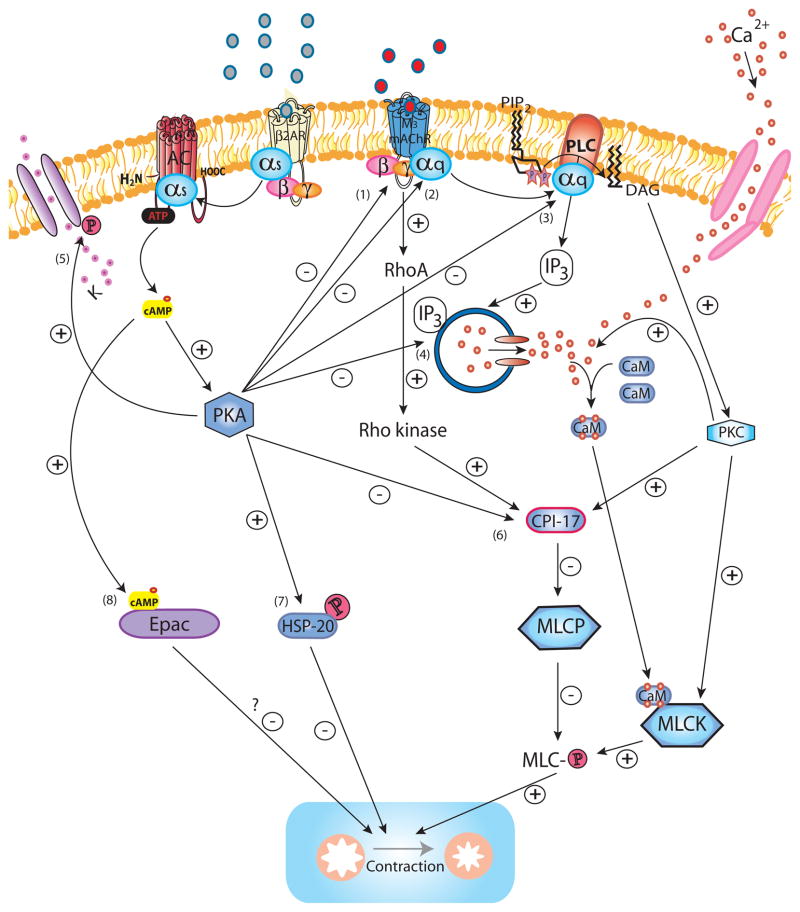
Figure 1. β2AR regulation of M3 mAChR signaling.
Upon activation of M3 mAChR by ligand binding, the Gαq subunit is released to bind and activate PLC, which hydrolyzes PIP2 into IP3 and DAG. IP3 induces Ca2+ release from intracellular stores via the IP3R whereas DAG activates PKC. The initial IP3-induced release of Ca2+, as well as cADP-ribose (not shown), both acting via RyR (not shown) contribute to a more sustained increase of intracellular Ca2+, also promoted by additional influx of Ca2+ from the extracellular space through Ca2+ channels on the cell membrane. Ca2+ binds calmodulin to form the Ca2+/calmodulin complex which activates MLCK to subsequently phosphorylate MLC allowing activation of myosin ATPase (not shown) which effectively leads to force generation by cross-bridge cycling (contraction). PKC promotes the influx of Ca2+ from the extracellular compartment by increasing the open probability of Ca2+ channels. PKC phosphorylation of the MLCP inhibitor CPI-17 mediates Ca2+ -sensitization as MLCP limits contraction by dephosphorylating MLC. Similarly, M3 mAChR activates RhoA and its downstream effector Rho kinase which also phosphorylates CPI-17 and thereby mediates Ca2+ -sensitization. PKC-mediated phosphorylation of MLCK may constitute an additional mechanism for Ca2+ -sensitization. Activation of the β2AR counteracts M3 mAChR-induced signaling and contraction at several levels. Ligand-induced conformation change of the β2AR results in the release of the Gαs subunit which subsequently activates AC to hydrolyze ATP to cAMP. cAMP binds to the PKA regulatory units (not shown) which then release the catalytically active PKA. PKA phosphorylates the M3 mAChR (1) (although relevance of this for desensitization in ASM is not certain), the Gαq subunit (2) and PLC (3) diminishing M3 mAChR activation and IP3 production (and possibly Rho kinase activation). The release of intracellular Ca2+ is inhibited through PKA-mediated phosphorylation of IP3R (4) and RyR (not shown), whereas phosphorylation of the KCa2+ (5) channels results in K+ efflux and hyperpolarization of the cell. PKA abrogates Ca2+ -sensitization by phosphorylating CPI-17 (6). PKA phosphorylation of HSP20 (7) renders it capable of inhibiting contraction through ill-defined mechanisms possibly involving regulation of actin dynamics and actin-myosin binding. Another potential effector downstream of cAMP, Epac (8), has been suggested as an inhibitor of contraction, however its role in ASM as an effector downstream of β2AR remains to be determined.