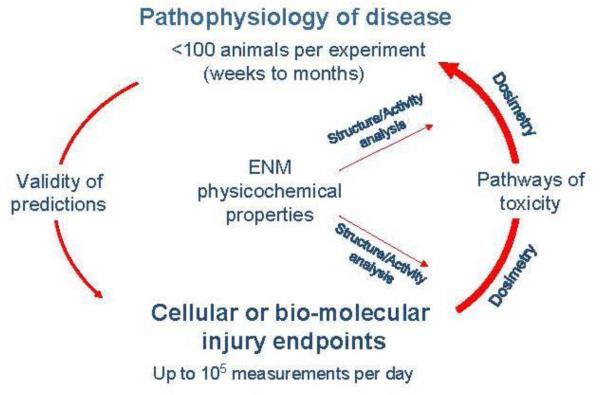
Fig. 1.
Principal components of the proposed predictive toxicological paradigm for engineered nanomaterial hazard assessment. A predictive toxicological approach is defined as establishing and using in vitro mechanisms and pathways of toxicity for predicting the pathophysiology of disease based on defined engineered nanomaterial physiochemical properties that engage the same toxicity pathways in vitro and in vivo. The in vivo outcome is used to validate the in vitro screening approach as being “predictive” and therefore appropriate for screening large batches of materials to obtain quantitative structure-activity relationships that also be apply to the in vivo outcomes. Accordingly, high-content and high-throughput screening in vitro can be used to determine priority of the in vivo approach, where fewer observations can be made owing to cost and time considerations. While the in vitro observations at this stage are not sufficient to drive the safety assessment, an established link to in vivo outcomes could accelarate the in vivo testing becauase of this approach, allowing the use of fewer animals.