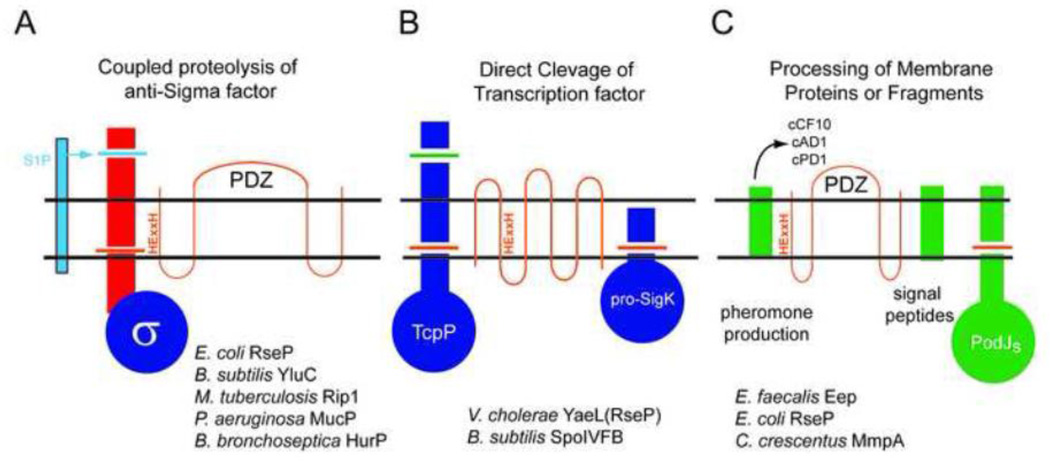
Figure 1. Conceptual Groupings of S2P mediated signaling Pathways.
A. Coupled Proteolysis of transmembrane anti-Sigma factors. In this widely distributed mechanism of ECF Sigma activation, an activating signal triggers Site one proteolysis (S1P, light blue) of the periplasmic domain of the anti-Sigma, which is rapidly followed by S2P mediated cleavage in the transmembrane segment (orange cut). The mechanism of this tight coupling is still poorly understood. The S2Ps of this type contain PDZ domains and include the S2Ps listed below the figure.
B. Direct Cleavage of a transcriptional regulator. In this mechanism, the proteolytic target is a membrane bound transcriptional regulator, either TcpP or pro-SigmaK. In the case of TcpP, which is cleaved by YaeL(RseP), the cleavage event shutsoff gene expression. In the case of pro-SigK, proteolysis (by SpoIVFB) liberates the active transcription factor. Note that the transmembrane topology pictured is that of SpoIVFB, not RseP (which is represented in Panel A).
C. Cleavage of membrane proteins or protein remnants. In this grouping, the S2P processes a membrane protein that is not a transcription factor. Examples include the production of peptide pheromones in E. faecalis from lipoprotein signal sequences by Eep, the processing of signal peptides by RseP, and the destruction of PodJs by MmpA in C. crescentus. The constitutive cleavage of FtsL by YluC also may fall into this category. See text and Tables for details.