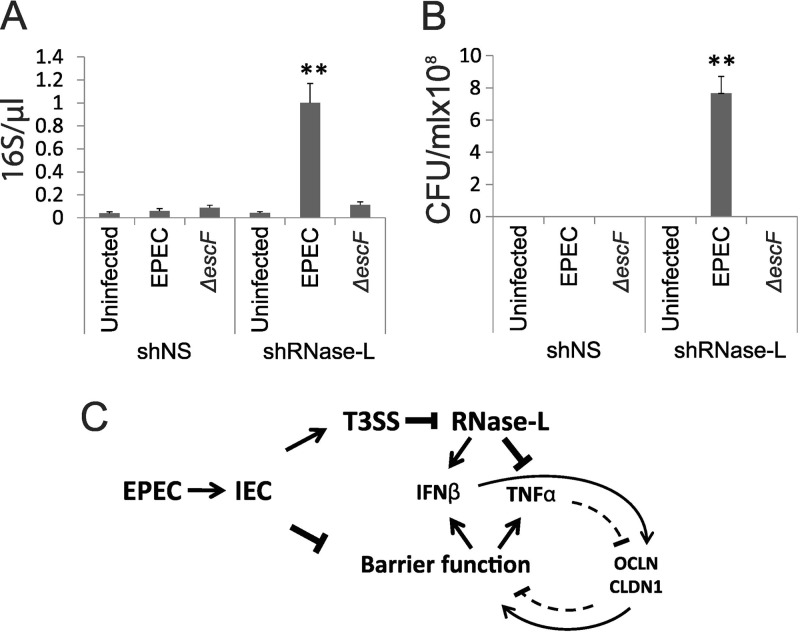
FIG 7.
RNase L deficiency increases Caco-2 monolayer permeability and bacterial translocation. (A and B) Differentiated shNS and shRNase L monolayers were infected with WT or ΔescF EPEC at an MOI of 50 for 3 h. The supernatants were collected from the basolateral chambers of transwell plates and analyzed by qRT-PCR to detect EPEC 16S rRNA (A) and by culturing overnight to determine the presence of viable bacteria (B). The data points shown are the means of 3 separate experiments. The error bars indicate standard deviations. P values were calculated using the Student t test. **, P < 0.01. (C) Model depicting the roles of IFN-β and RNase L in the regulation of IEC barrier function following EPEC infection. EPEC infection disrupts barrier integrity, leading to activation of PRRs and induction of cytokines, including IFN-β and TNF-α. Injection of T3SS effectors, including nleD, inhibits RNase L, leading to diminished induction of IFN-β and enhanced TNF-α. The opposing effects of these cytokines on IEC barrier function occur through cytokine-mediated regulation of the TJ proteins OCLN and CLDN1. Additional mechanisms that contribute to the regulation of cytokine induction and barrier function are discussed in the text.