FIG 1.
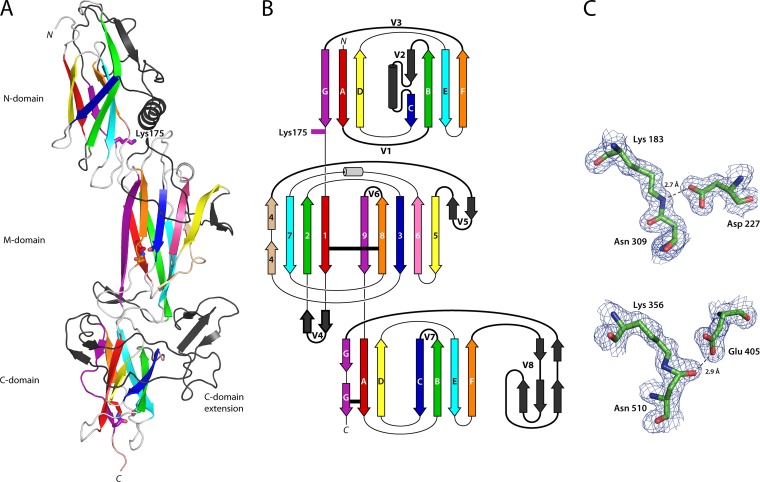
Structure of the T6 backbone pilin. (A) Ribbon diagram depicting the three domains of T6 colored as in the topology diagram (B) from red (N terminus) to purple (C terminus). Each domain is decorated with extended loops or variable regions (colored black, V1 to V8), the largest in the C domain is termed the C-domain extension. Residues involved in isopeptide bond formation and the N-domain pilin lysine (Lys175) are shown in stick form, colored the same as the β-strand from which they originate. (B) Topology diagram color-coded the same as the ribbon diagram. CnaB domain β-strands are labeled A to G, while the CnaA domain β-strands are labeled 1 to 9. The variable loop regions connecting β-strands are shown as black, labeled V1 to V8. Isopeptide bond position is depicted as horizontal black lines. (C) Residues involved in isopeptide bond formation in the M domain (top) and C domain (bottom) are shown in stick form, colored by atom type, in electron density from a 2Fo-Fc map contoured at 0.38 eÅ−3 (1.2σ). Hydrogen bonds are shown as broken lines.