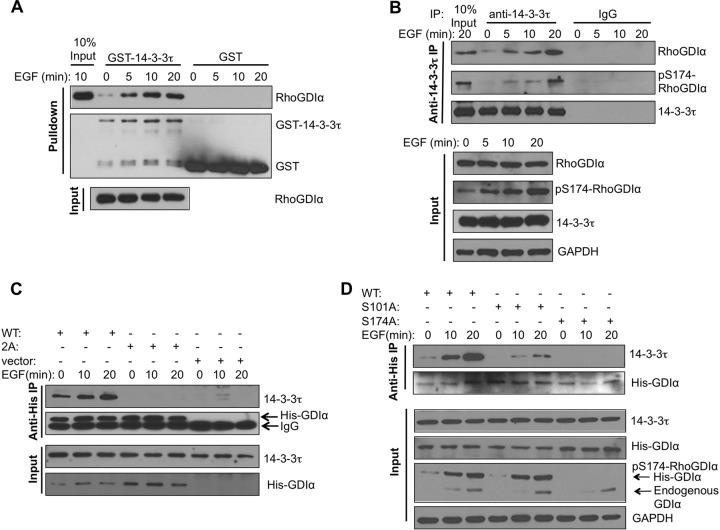
FIG 4.
14-3-3τ binds RhoGDIα at phosphorylated S174 and activates Rho GTPases. (A) In vitro interaction between purified GST–14-3-3τ and RhoGDIα derived from EGF-treated cells. Starved MCF7 cells were stimulated with EGF for the indicated times. Cell lysates were then incubated with GST- or GST–14-3-3τ-conjugated glutathione-Sepharose beads. The amounts of cellular RhoDGIα pulled down by GST–14-3-3τ and total RhoGDIα present in MCF7 cell lysates were determined by Western blotting. (B) 14-3-3τ associated with S174-phosphorylated RhoGDIα upon EGF treatment in MCF7 cells. Endogenous 14-3-3τ was immunoprecipitated (IP) from the EGF-treated MCF7 cell lysates, followed by immunoblotting to detect the coimmunoprecipitated RhoGDIα and S174-phosphorylated RhoGDIα. Total cell lysates were subjected to immunoblotting to determine the amounts of input proteins (lower). (C) Double mutations of the Ser101/Ser174 residues abolished EGF-induced interaction between 14-3-3τ and RhoGDIα. MCF7 cells were transfected with His-tagged wild-type (WT) or S101A/S174A mutant (labeled as 2A) RhoGDIα. Cells were treated with EGF, and cell lysates were harvested for immunoprecipitation with anti-His antibody, followed by immunoblotting with the antibody specific to 14-3-3τ. Immunoblotting was also performed to determine the input levels of 14-3-3τ and His-tagged RhoDGIα in whole-cell lysates. (D) Mutation of Ser174 of RhoGDIα to Ala abolished its interaction with 14-3-3τ. MCF7 cells were transfected with either His-tagged WT or S101A or S174A mutant RhoGDIα. Cells were starved overnight and then treated with EGF. Cell lysates were subjected to coimmunoprecipitation to determine the association between His-RhoDIα and endogenous 14-3-3τ as described for panel C. Immunoblotting was also performed to determine the input levels of 14-3-3τ, endogenous and His-tagged RhoGDIα, pS174-RhoGDIα, and GAPDH in the whole-cell lysates.