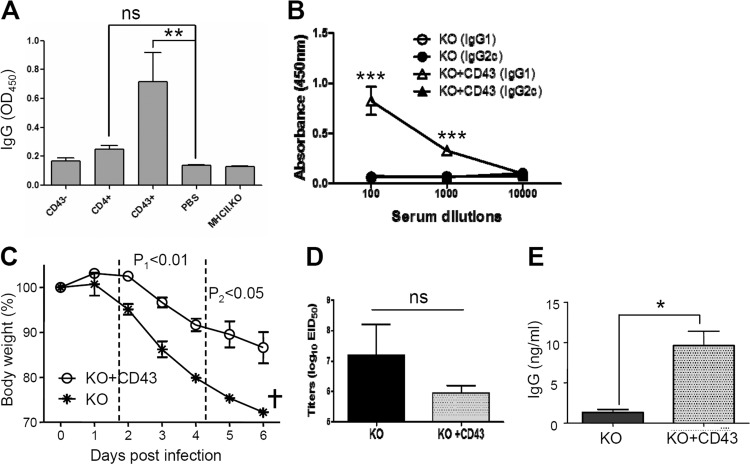
FIG 7.
Adoptive transfer of B6 WT fractionated CD43+ cells to MHC-II KO mice confers protective immunity. (A) Vaccine-specific IgG antibody responses in MHC-II KO mice with adoptive transfer. Naive MHC-II KO mice (n = 4 for each group) received CD43−, CD4+, and CD43+ cell populations fractionated from spleens of naive B6 WT mice. CD43−, 3.3 × 107 enriched CD43− cells per MHC-II KO mouse; CD4+, 1.5 × 107 enriched CD4+ T cells per MHC-II KO mouse; CD43+, 2.2 × 107 enriched CD43+ cells per MHC-II KO mouse; PBS, no B6 WT cells (a negative control); MHCII.KO, a serum ELISA control from MHC-II KO mice immunized with influenza VLP vaccine. (B) IgG isotypes (IgG1 and IgG2c) in immune sera of MHC-II KO mice with adoptive transfer of naive wild-type CD43+ cells after influenza VLP immunization. MHC-II KO mice with or without CD43+ adoptive transfer were intramuscularly immunized with influenza A/PR8 VLPs. KO, MHC-II KO mice without adoptive transfer prior to immunization; KO+CD43, MHC-II KO mice with CD43+ cell adoptive transfer prior to immunization. (C to E) Protective roles of wild-type CD43+ cells in MHC-II KO mice. MHC-II KO mice with (KO+CD43) or without (KO) CD43+ adoptive transfer were intramuscularly immunized with influenza A/PR8 VLPs and then challenged with influenza virus 2 weeks later. (C) Body weight changes. (D) Lung viral loads. (E) Virus-specific IgG antibodies in BALF. ns, not significant; †, all mice were euthanized. Statistical significances were compared between KO and KO+CD43: *, P < 0.05; **, P < 0.01; ***, P < 0.0001.