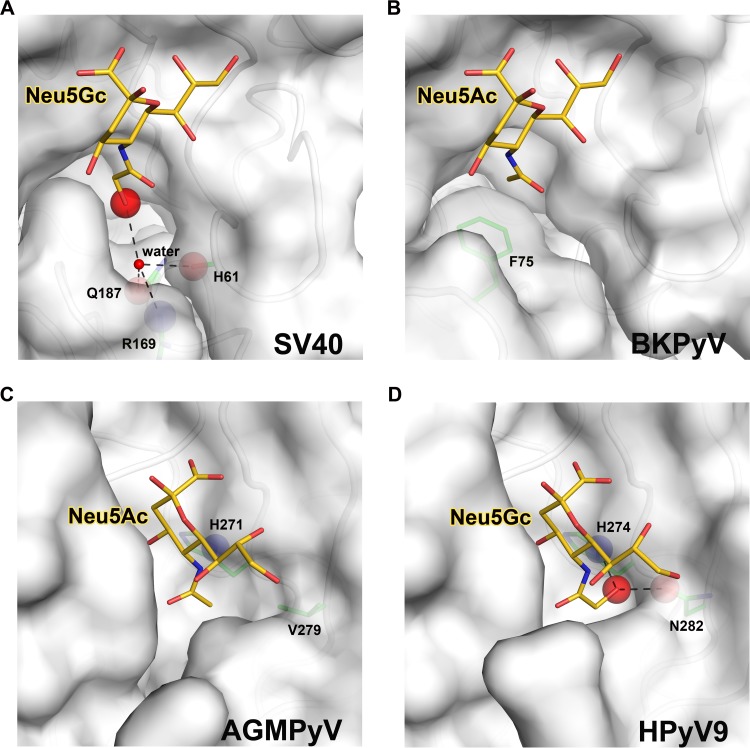
FIG 2.
Sialic acid-binding sites in human and simian polyomaviruses. Views into the receptor-binding pockets of SV40 (A), BKPyV (B), AGMPyV (C), and HPyV9 (D) VP1. The sialic acid receptors (Neu5Ac or Neu5Gc) bound by each structure are shown in stick representation and colored gold (carbons), blue (nitrogens), and red (oxygens). The extra oxygen of Neu5Gc is highlighted with a red sphere. VP1 residues that define the specificity for either type of sialic acid are shown as sticks and colored green (carbons), blue (nitrogens), and red (oxygens). The protein main chain or side chain atoms interacting with the extra oxygen of Neu5Gc are highlighted as blue spheres for nitrogens or red spheres for oxygens. Hydrogen bonds are shown as black dashed lines. (A) Model of SV40 VP1 in complex with Neu5Gc (modified from PDB ID 3BWR). Neu5Gc can easily be accommodated in the conformation shown, and the extra oxygen of Neu5Gc could make favorable interactions with several side chains. (B) Structure of BKPyV VP1 in complex with Neu5Ac (modified from PDB ID 4MJ0). The presence of F75 prevents BKPyV from accommodating Neu5Gc. (C) Structure of AGMPyV VP1 in complex with Neu5Ac (modified from PDB ID 4MBZ). The binding site is less polar and does not favor an interaction with Neu5Gc. (D) Structure of HPyV9 VP1 in complex with Neu5Gc (modified from PDB ID 4POT). HPyV9 has enhanced specificity for Neu5Gc due to contacts of the extra hydroxyl group with two polar side chains.