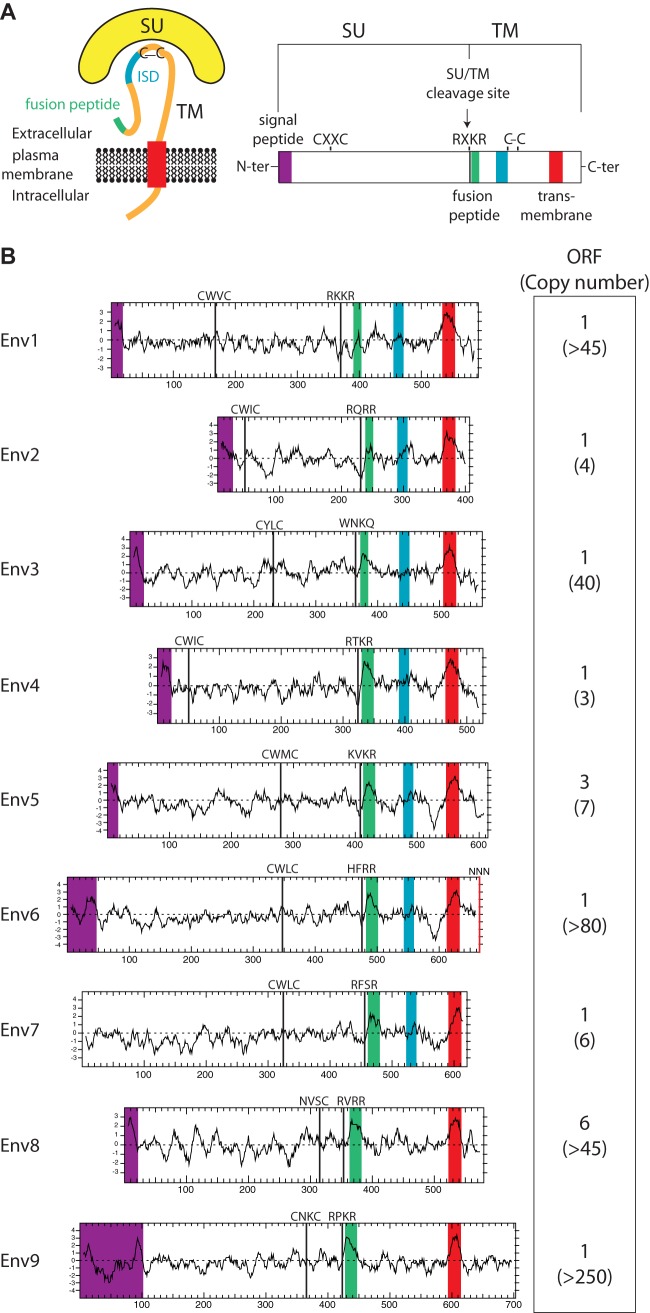
FIG 2.
Structure of a canonical retroviral envelope protein and characterization of the identified Ictidomys tridecemlineatus candidates. (A) Schematic representation of a retroviral envelope protein, with the surface (SU) and transmembrane (TM) subunits delineated, and the furin cleavage site (consensus, R/K-X-R/K-R) between the two subunits together with the C-X-X-C domain involved in SU-TM interaction indicated; the hydrophobic signal peptide (purple), fusion peptide (green), transmembrane domain (red), and putative immunosuppressive domain (ISD) (blue) are also indicated. (B) Characterization of the thirteen-lined ground squirrel candidate envelope proteins. (Left) The hydrophobicity profile for each candidate is shown with the positioning of the canonical structural features schematized in panel A, when present (same color code). (Right) Number of full-length env gene ORFs within each family of element and total number of genomic copies (in parentheses).