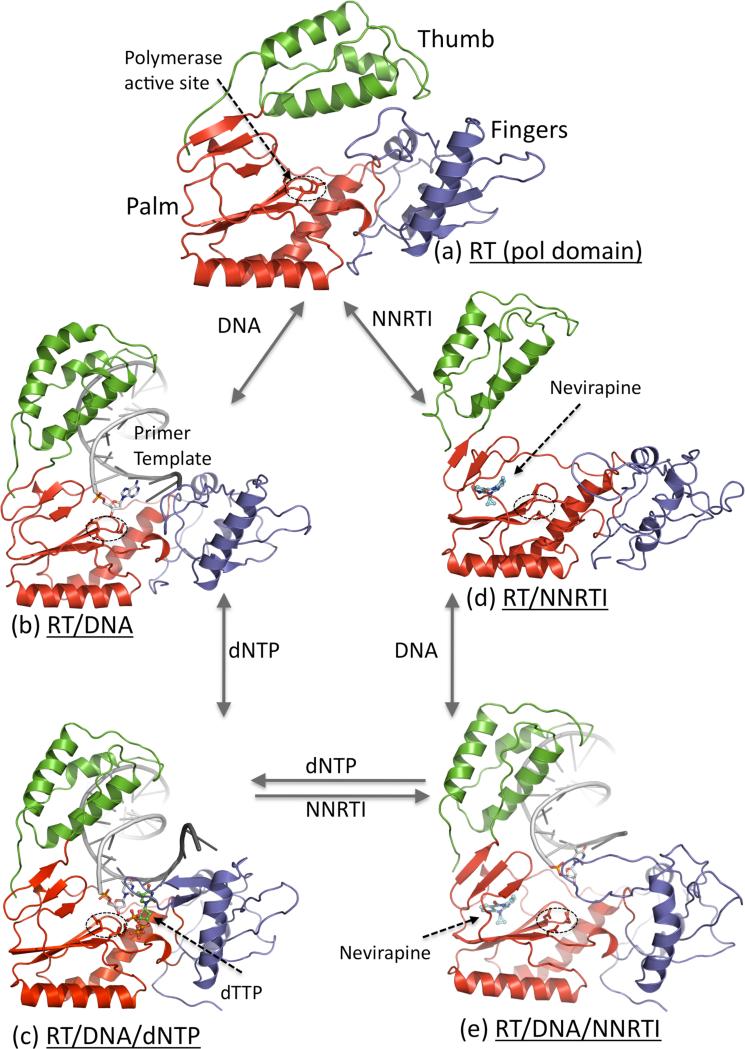
Figure 2.
Five structurally characterized conformational states of HIV-1 RT; only the polymerase domain (fingers (blue), palm (red), and thumb (green)) of RT is shown. (a) Thumb is positioned near the fingers in closed conformation that occupies the nucleic acid binding cleft in apo HIV-1 RT structures [29]. (b) Upon binding of a nucleic acid substrate, the thumb is lifted up and acts as a clamp to hold the nucleic acid [14]; the primer 3′-end is positioned at the polymerase active site (D110, D185, and D186) that is highlighted by a dotted ellipse. (c) Binding of a dNTP to RT-DNA complex results in closing of the fingers to bind a dNTP in a catalytically competent state [30]. (d) Binding of an NNRTI to RT causes several conformational changes; thumb subdomain is lifted to a hyper-extended position and the nucleic acid-binding cleft is open even in absence of a nucleic acid [13]. (e) Binding of an NNRTI to RT-DNA complex resulted in reduction of DNA interactions with the polymerase domain of RT, and repositioning of the primer 3′-end away from the polymerase active site [31••]; the structural study revealed no ordered dNTP binding to RT-DNA-NNRTI complex.