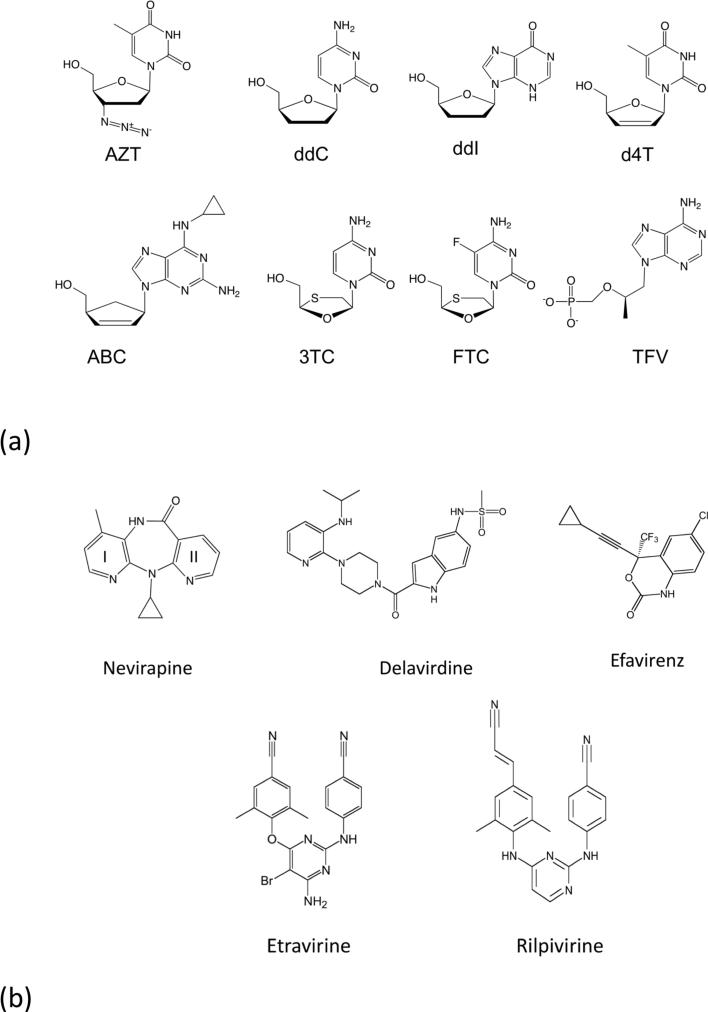
Figure 3.
The chemical structures of anti-AIDS drugs that target HIV-1 RT: (a) The clinically approved NRTIs are: (i) AZT (zidovudine, ZDV, azidothymidine, Retrovir®) has a 3′-azido group substituted for the 3′-OH of dTTP; (ii) ddC (dideoxycytidine, zalcitabine, Hivid®) (iii) ddI (didanosine, Videx®), and (iv) d4T (stavudine, Zerit®) lack 3′-OH groups; (v) ABC (abacavir, Ziagen®) has a cyclopentene ring, (vi) 3TC (lamivudine, Epivir®) has an altered β-L-pseudo-ribose ring, (vii) FTC (emtricitabine, Emtriva®), and (viii) TFV (tenofovir) is a nucleotide analog that has an acyclic methoxypropyl moiety substituted for the deoxyribose ring of dATP; tenofovir is formulated as tenofovir disoproxil fumarate (TDF, PMPA, Viread®). (b) Chemical structures of the five NNRTI drugs approved for treating HIV-1 infections. (i) Nevirapine (NEV, Viramune®), (ii) delavirdine (DLV,Rescriptor®), (iii) etravirine (ETR, Intelence®), and (iv) rilpivirine (RPV, Edurant®).