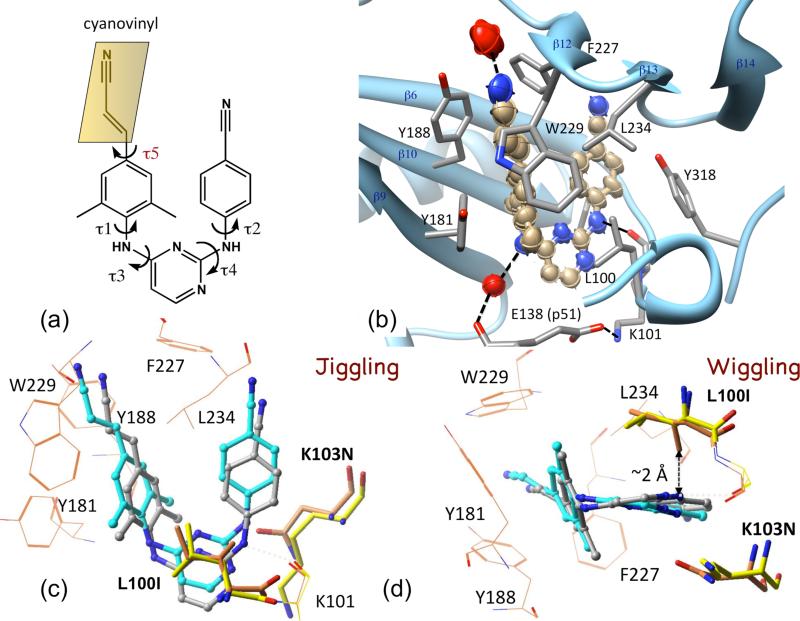
Figure 4.
Wiggling and jiggling of an NNRTI to retain potency against drug-resistance mutations. (a) Chemical structure of rilpivirine; the five torsionally flexible bonds define the conformational freedom of the NNRTI. (b) Thermal ellipsoid representation of rilpivirine drawn using the anisotropic B-factors of individual atoms from 1.5 Å resolution structure of RT-rilpivirine complex (PDB ID 4G1Q) [25•]. The interacting side chains and water molecules (red) are displayed. (c & d) Comparison of the binding modes of rilpivirine to wild-type RT (gray) vs. L100I + K103N mutant RT (cyan) revealed how the drug jiggle and wiggles, respectively, to evade the effects of drug-resistance mutations [23•]; the mutations modifies the side chains from yellow to orange.