Table 3.
Intermolecular carboetherification/Heck-type couplings[a]
| Entry | Substrate | Alkene | Product | Yield (%)[b] | ee (%)[c] |
|---|---|---|---|---|---|
| 1[d] |
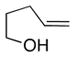 12 |
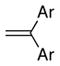 13a Ar = Ph |
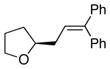 14 |
92 | 82 |
| 2 |
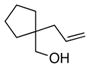 15 |
13a |
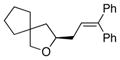 16 |
90 | 82 |
| 3 |
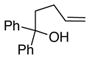 17 |
13a |
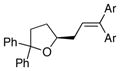 18a, Ar = Ph |
90 | >95 |
| 4 | 5 | 13a |
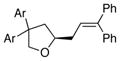 19, Ar = 4-ClC6H4 |
80 | 80 |
| 5 |
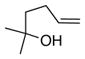 20 |
13a |
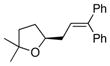 21 |
84 | >95 |
| 6 |
22 |
13a |
23/24 (dr = 6:1) |
90 | 86 (maj) >95 (min) |
| 7 | 17 |
13b Ar = 4-MeOC6H4 |
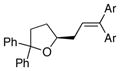 18b Ar = 4-MeOC6H4 |
88 | >95 |
| 8 | 17 | 13c, Ar = 4-F-C6H4 | 18c, Ar = 4-F-C6H4 | 70 | >95 |
| 9 | 17 |
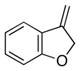 25 |
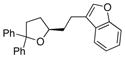 26 |
88 | 94 |
| 10 | 17 |
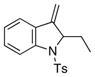 27 |
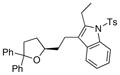 28 |
81 | nd[e] |
| 11 | 17 |
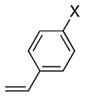 29a X = OMe |
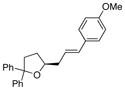 30, E:Z = >20:1 |
46 | >95 |
| 12 | 20 |
29b X = t-Bu |
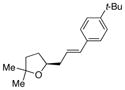 31, E:Z = >20:1 |
42 | 95 |
Reactions were run under anhydrous conditions under Ar in a sealed tube. 20 mol% of Cu(OTf)2 was complexed with 25 mol% (S,S)-t-Bu-box (60 °C for 2 h in 1 mL PhCF3) then ca. 0.145 mmol alkenol substrate in PhCF3 (0.1 M total), 3 equiv vinylarene, 1 equiv K2CO3, 3 equiv MnO2 and ca. 36 mg 4 Å mol sieves were added and the reaction stirred at 100 °C for 16 h unless otherwise noted.
Isolated yield following chromatography on silica gel.
Enantiomeric excess determined by chiral HPLC.
Reaction concentration of 0.08 M used.
Not determined. Enantiomers would not separate on several chiral HPLC columns.
