FIGURE 2.
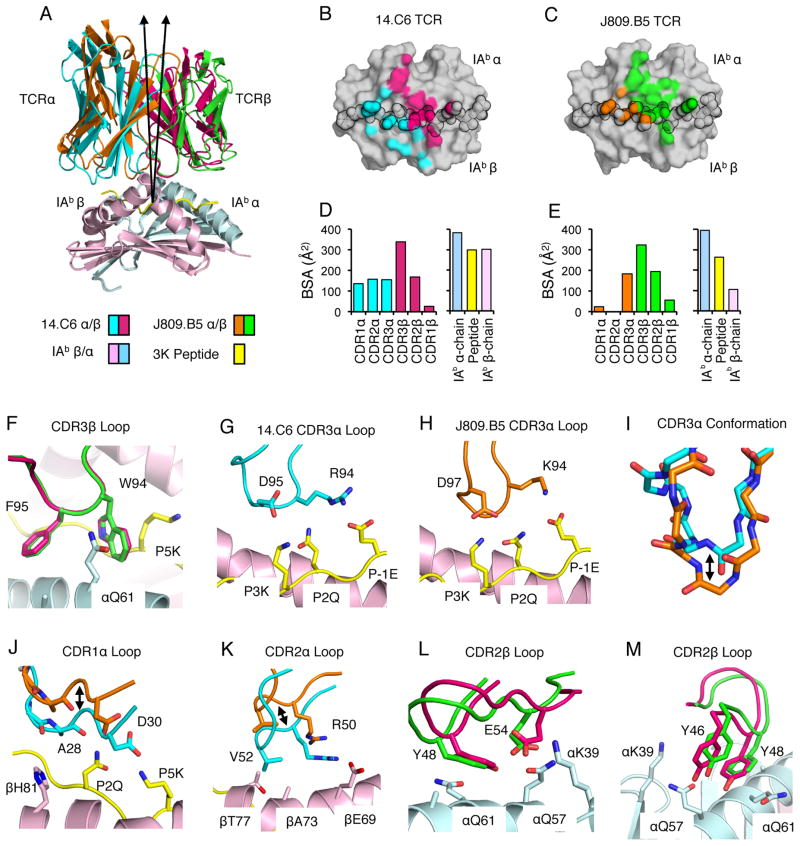
CDR3α Sequence Can Alter the Positioning of CDR1 and CDR2 Loop Atop MHC. (A) Overlay of the 14.C6 and J809.B5 TCRs binding IAb-3K. The 14.C6 TCR is colored red (TCRβ) and blue (TCRα); the J809.B5 TCR is colored green (TCRβ) and orange (TCRα). IAb-3K is colored cyan (IAbα chain), yellow (peptide), and magenta (IAbβ chain). A 7.5% difference in the tilt of the TCR docking geometry atop IAb-3K is observed based on a vector from the center of mass of each TCR to the α-carbon of the p5 peptide residue. (B) Projection of the 14.C6 TCR or (C) J809.B5 TCR binding onto IAb-3K. 14.C6 TCRα contacts are colored blue, 14.C6 TCRβ contacts are colored red. The J809.B5 TCRα contacts are colored orange and the TCRβ contacts are colored green. The peptide residues are outlined in black. (D) The amount of buried surface area (BSA) contributed by the 14.C6 or (E) J809.B5 TCRα, TCRβ, peptide or MHC chains for the binding reaction with IAb-3K. (F) The 14.C6 CDR3β loop (magenta) and the J809.B5 CDR3β loop (green) are in a similar conformation and make similar contacts with IAb-3K. (G) The 14.C6 CDR3α loop residues R94 and D95 interact with the peptide residues, P-1E, P2Q and P3K. (H) The J809.B5 CDR3α loop residues K94 and D97 interact with the peptide residues, P-1E, P2Q and P3K. (I) The 14.C6 CDR3α loop (blue) and the J809.B5 CDR3α loop (orange) are in a different conformation when bound to IAb-3K. (J) 14.C6 CDR1α residues A28 and D30 (blue) make extensive contacts with the P2Q and P5K residues of the peptide, and the IAbβ chain residue H81. The J809.B5 CDR1α loops residues A28 and D30 (orange) are shifted approximately 4 Å, allowing only the A28 residue to make minimal contacts with peptide, and no contact with MHC. (K) The 14.C6 TCR CDR2α residues R50 and V52 (blue) interact with IAb β-chain residues βE69, βA73 and βT77, contacts that are not present in the J809.B5:IAb-3K structure (orange) due to a 4 Å rigid body shift. (L, M) The main-chain of CDR2β loop at the βY48 position in the 14.C6 structure (green) is shifted approximately 1Å as compared to the J809.B5 structure (magenta), and some rotamer differences are observed for the CDR2β residues E54 and Y46. Figures were made with PyMol (76).